
The endoscopic brain robot’s arms are each 2.8 mm in diameter and 35 mm long when fully extended. Together, they offer a 43 mm diameter workspace. [Image courtesy of Boston Children’s Hospital]
Researchers say they’ve developed a surgical robot for removing brain tumors in children, and that it could also offer a less invasive, safer option for adult neurosurgery and other procedures.
The trick is using hollow, nitinol robot arms to allow neurosurgeons to swap tools during a tumor resection procedure, said Pierre DuPont. He’s the chief of pediatric cardiac bioengineering at Boston Children’s Hospital, and the corresponding author of a new research paper detailing the potential advantages of a two-armed neuroendoscopic robot.
In an interview with Medical Design & Outsourcing, DuPont traced the project back to conversations with Dr. Jim Drake, chief of neurosurgery at SickKids in Toronto, sometime around 2016. DuPont had been looking for clinically relevant applications for surgical robotics and had homed in on neurosurgery. Drake laid out two main requirements for a surgical robotics system to help remove pediatric brain tumors: a large workspace at the tip of the trocar and the ability to swap tools while keeping the robotic arms in place.
DuPont remembers Drake telling him, “I want to have my arms in there, my scope in there — I don’t want to remove any of that. It’s too dangerous, I just want to be able to swap a tool. I may have six different types of forceps, six different types of scissors, bipolar forceps to control the bleeding. And I don’t want to move anything while I’m swapping these in and out.”
Developing the robotic arms
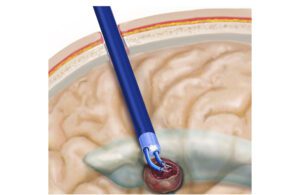
The endoscopic robot could improve the safety and effectiveness of brain tumor removal. [Illustration courtesy of Boston Children’s Hospital]
DuPont worked with mechanical engineers at Boston Children’s Hospital: Joseph Peine, who is now at Vicarious Surgical, and Karl Price, now at Boston Dynamics. They won a grant from the National Institutes of Health to fund development of their tool-swapping robot.
“We needed to use basically hollow arms,” DuPont said. “There were a few technologies that would give you that: tendon actuation like in steerable catheters, the same technology that’s used in robotic bronchoscopes. And then there’s another technology — concentric tube robots, which is something that I developed when I was at Boston University, and also other folks have worked on it in a contemporaneous fashion.”
Those concentric tubes are made of nitinol and pre-curved, with the curvature set using the nickel-titanium alloy’s superelastic properties.
“When you slide one inside the other, if the curvatures are opposing each other they straighten each other out,” DuPont said. “And then if you just rotate them, they take that bent shape, so you get this trumpet-shaped workspace that you can rotate around.”
The pre-curved nitinol tubes deliver enough rigidity to handle the forces of surgery without buckling to give surgeons more room to work.
“The technological jump here is how can we make concentric tube robots with bigger workspaces, so they bend more left to right, but also perfectly balance. … The real limitation is workspace size, so we developed a technique to increase that size,” DuPont said.
The solution was a laser-cut pattern on the nitinol tubes. Such an approach has been considered to enhance stability, but DuPont’s team also used the diamond-shaped holes cut through the wall of the tube to expand the field within which a neurosurgeon could work.
“We discovered that instead of using a solid tube, if we laser pattern the tubes in the right way, we could pre-curve them with a much tighter curvature without them buckling. And we can also use that pattern to control the relative stiffness of the tubes,” DuPont said. “When you have those two tubes and the curvatures are fighting each other, if they’re not exactly equal, the thing won’t be straight, it’ll be a little bent and then you have a hole in the middle of your workspace. And so by controlling that pattern, we can arrange it so they exactly straighten out.”
Testing the brain robot’s system
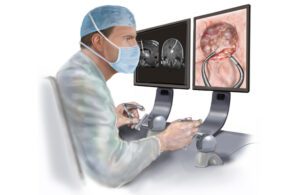
The robotic endoscope has a a 2.4 mm diameter aspiration/irrigation channel for removing tumor fragments, while a 4.2 mm diameter chip camera with LED illumination allows the surgeon to see the procedure. [Illustration courtesy of Boston Children’s Hospital]
The researchers’ paper compares the robotic technology for brain tumor resection against traditional open-brain procedures and endoscopic surgery.
“Right now, 90% of the surgeries are done as open surgery. You’re viewing through a microscope, you push the good brain away to get to the bad brain. And then endoscopic, you’re doing this one-handed, one-tool-type of removal,” DuPont said. “With robotic, you’ve got got the best of both worlds: both arms for open surgery and a variety of tools, but minimal [invasiveness].”
DuPont also works in the cardiac surgery space for procedures such as catheter-based valve repair, so when he wants to test one of those devices, he heads to the pig hearts in the animal labs.
“Pigs have hearts that are the same size as humans, but for brains, that doesn’t really exist. And I don’t want to do [tests with] chimpanzees or dogs or whatever,” DuPont said.
Instead, they devised a set of skill tasks — like those used to train and qualify laparoscopic surgeons — to evaluate their prototypes, using ripe bananas to mimic brain tumors.
“Tumors in brains can have various consistencies. Some of them are very soft, and you can actually just suck them out. Others need to be torn apart — they’re fibrous,” DuPont said. “When we were trying to come up with the models for a tumor, we went to the grocery store to the produce department, and we just bought every fruit. We were trying strawberries and blueberries. We needed something that would be consistent. And it turned out that bananas were the fruit of choice.”
What’s next?

Pierre DuPont is the chief of pediatric cardiac bioengineering at Boston Children’s Hospital. [Photo courtesy of Boston Children’s Hospital]
DuPont has a team of Babson College MBA students investigating the commercialization of the system. He said he’s also had discussions with major device manufacturers, but it’s too early for strategic investors.
“They’ll joke about it: they would rather pay hundreds of millions for something that’s gone through first-in-human trials and overpay rather than take a risk early,” DuPont said.
The system could have applications beyond neurosurgery, such as transurethral procedures for benign prostatic hyperplasia or transvaginal procedures for uterine fibroids.
Previously: Boston Children’s to conduct FDA-approved studies of heart valve that grows as children do