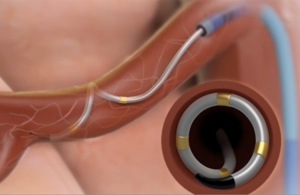
The Symplicity Spyral renal denervation system delivers radiofrequency energy via a catheter to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
Analysts expect good news for Medtronic and its renal denervation (RDN) therapy for hypertension when the FDA review panel meets later this month.
RDN therapy could be a life-saver for millions of patients with high blood pressure — and a billion-dollar business for Medtronic if it wins FDA approval after two failed trials in the past decade.
The world’s largest medical device company is looking for a win after the latest RDN pivotal trial fell short of expectations, a disappointing result in a year with many other challenges. Medtronic’s revenue slipped 1% in fiscal 2023 (which ended on April 28, 2023), and net income dropped by 25% year-over-year as the company cut costs with early retirements, layoffs and operating unit restructuring.
FDA approval of Medtronic’s Symplicity Spyral RDN technology could take some of the sting off problems across the company’s diversified businesses, including the long struggle to remediate diabetes product safety problems and, most recently, a massive implantable defibrillator recall.
Related: Medtronic CEO Geoff Martha offers updates on supply chain, labor, R&D, Hugo and more
Needham analysts predict the panel will support Medtronic RDN
“RDN could eventually become a meaningful growth driver for [Medtronic] if its Symplicity Spyral system is approved by the FDA. … Overall, we think it’s likely that the panel will support Symplicity Spyral’s safety and efficacy,” Needham analysts Mike Matson, David Saxon and Joseph Conway wrote in a company update.
They think the panel may focus on the latest pivotal trial’s failure to meet its primary efficacy endpoint, as well as whether the blood pressure improvement justifies the procedure, whether RDN can lower the rate of cardiovascular events, which patients are most likely to benefit, appropriate indications and longer-term safety and durability.
It’s too soon to tell on that final point, the analysts said, and they don’t envision any of the other topics becoming deal-breakers for the committee.
While Medtronic’s trial didn’t show a statistically significant difference in ambulatory blood pressure reduction in ambulatory systolic blood pressure for RDN versus the sham procedure, there was a statistically significant difference in office-based systolic blood pressure reduction. And the device-maker has offered some possible explanations for failing to meet the primary efficacy endpoint. Considering all of this, the Needham analysts expect the panel will “ultimately overlook the fact that the trial missed its primary efficacy endpoint.”
They also note that Symplicity Spyral RDN reduces blood pressure by around 5-10 mm Hg, and that Medtronic has said a reduction of just 2-5% mm Hg can reduce cardiovascular events by 10%.
“And while drugs are effective, patient compliance is a problem with only one of four people having their hypertension under control and 50% of patients stopping drugs within one year,” the analysts wrote. “While the panel may express some disappointment that RDN doesn’t result in larger blood pressure reductions, we expect them to ultimately support the view that RDN has a place in the treatment paradigm, especially considering the poor compliance with drugs.”
Who benefits from RDN?
An estimated 1.3 billion people suffer from hypertension worldwide, but only around 40% are being actively treated. Of those, only around 20% feel like their symptoms are under control.
Expect the panel to discuss whether Symplicity Spyral RDN should be indicated only for patients with severe uncontrolled hypertension, how exactly a patient should qualify and whether other cardiovascular risks should factor in.
Needham also notes that some RDN patients experience very large blood pressure reductions, while others experience very small reductions. Rochester, Minnesota–based Geneticure says it can test patients to determine which patients will see the most benefit, and also to flag patients whose blood pressure might spike after treatment.
“While it’s exciting for market adoption that Geneticure can augment the RDN drop in blood pressure to levels that are clinically meaningful, the company is most excited to be able to identify patients that could have a dangerous increase in blood pressure post-denervation,” Geneticure co-founder and CEO Scott Snyder said in a news release after presenting data at the 2022 American Heart Association Scientific Sessions.
The Mayo Clinic-backed biotech firm has taken aim at Medtronic’s study, saying the placebo effect is likely responsible for RDN results after patients learned they received the treatment, and that Medtronic’s interpretation of the data may have artificially increased 36-month results.
The Needham analysts said they think Medtronic will “argue that there is no reliable way to identify responders and that it should be available to all properly indicated patients.”
Save the date for FDA’s RDN panel
The FDA Medical Devices Advisory Committee’s Circulatory System Devices Panel will meet Wednesday, Aug. 23 to discuss and vote on Medtronic’s pending premarket approval (PMA) request for its Symplicity Spyral Renal Denervation System.
The panel will also meet one day earlier — Tuesday, Aug. 22 — to consider and vote on a PMA submission from Otsuka Medical Devices subsidiary ReCor Medical for its Paradise Ultrasound RDN system.
The presentations will be held virtually from 9 a.m. to 6 p.m. Eastern on both days.
Related: 5 growth areas where Medtronic’s CEO wants to invest more