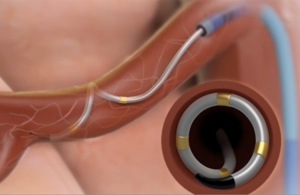
Medtronic’s Symplicity Spyral renal denervation catheter delivers energy to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
Medtronic faced tough questions about its Symplicity Spyral renal denervation (RDN) therapy for hypertension at today’s FDA review panel.
Medtronic is seeking approval of its Symplicity Spyral multi-electrode RDN catheter and Symplicity G3 radiofrequency generator “for the reduction of blood pressure in patients with uncontrolled hypertension despite the use of anti-hypertensive medications or in patients in whom blood pressure lowering therapy is poorly tolerated.”
The stakes are high for Medtronic, which has been developing the technology for years in the belief that it could be a billion-dollar business and provide relief to millions of patients worldwide, reducing heart attacks, strokes and other serious events tied to high blood pressure.
The same FDA review panel yesterday supported approval of competing RDN technology developed by Otsuka Medical Devices’ ReCor Medical. If the FDA agrees with the panel’s recommendation on the safety and efficacy of ReCor’s system, it could be the first RDN therapy to be approved for use in the U.S.
But ReCor’s clinical trial met its primary endpoint for efficacy, while Medtronic’s RDN trials have failed to hit that target — though analysts have said they don’t think that will prevent Medtronic from winning FDA approval.
More than 1 billion people suffer from hypertension globally, but only around 40% are receiving active treatment. Of those, around one-fifth report feeling like their symptoms are under control.
Medtronic has said its Symplicity Spyral RDN reduces blood pressure by around 5-10 mm Hg, and that a reduction of just 2-5% mm Hg can reduce cardiovascular events by 10%.
Related: Pay drops for Medtronic CEO and the median employee; bonus plan changes
FDA review panel questions Medtronic on RDN

Medtronic’s Symplicity Spyral device is a multi-electrode radiofrequency catheter. [Image courtesy of Medtronic]
In today’s FDA review panel, experts in medicine and statistical analysis questioned Medtronic about its latest RDN trial design, data, analysis and findings.
Ben Saville, director and senior statistical scientist at Berry Consultants, said he believed Medtronic “introduced some bias in this analysis” but that it does not likely affect the high-level conclusions of the study.
Reviewers expressed concerns that study outcomes may have been influenced by analytical bias and that blinded patients may have determined whether they received the RND therapy. One reviewer called attention to a “lack of race and other demographic information.”
“That certainly would be a big problem for publishing in most of our peer-reviewed journals in this day and age, especially for a question where there’s so much relevance of race and ethnicity to the clinical problem,” said Dr. Matthew Corriere, professor of cardiovascular surgery at the University of Michigan.
“The effect of renal denervation in the African-American population is an important one to consider,” added Dr. Bram Zuckerman, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health.
Dr. Eric Bates, a cardiology professor at the University of Michigan, asked why Medtronic has not yet published its data despite presenting results at the American Heart Association’s meeting in November.
Vanessa DeBruin, senior director of clinical research at Medtronic, said the paper is still in review.
“The hard question is why was it not a simultaneous publication and why nine months later have we not seen the data published?” asked Bates, later continuing, “My concern is had the report been published, the spin on the technology in the last six months in preparation for this meeting might have been muted compared to what I’m reading in the lay and medical press.”
As of publication, the FDA review panel had taken its lunch break. Medtronic will address the reviewers’ questions this afternoon, with a discussion and vote by the panel to follow. We’ll update this story later today.
Related: 5 growth areas where Medtronic’s CEO wants to invest more