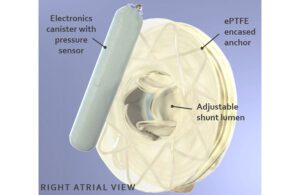
Adona Medical is designing its adjustable heart shunt device with pressure sensors on both sides of the heart. [Illustration courtesy of Adona Medical]
Medical devices have yet to tap nitinol’s shape memory properties, but that’s just one breakthrough Adona Medical hopes to achieve with its adjustable, bi-atrial-sensing heart shunt.
Adona Medical co-founder and CEO Brian Fahey’s presentation on his shunt device startup’s aspirations elicited palpable interest from the cardiologists in the room at CSI Frankfurt.
“You plan to disrupt two fields in heart failure?” asked Dr. Daniel Burkhoff, the director of heart failure, hemodynamics and MCS research at the Cardiovascular Research Foundation’s Clinical Trials Center.
“We’re going to try,” Fahey replied. “Our plan is to improve patient care.”
Fahey has publicly said little about the cardiac device developer’s technology outside of that 10-minute presentation. But if the Adona Medical team can achieve only half of their intent, it could mark progress not only in cardiac care but in the use of nitinol’s shape memory properties.
“We’re trying to bring two really key technologies to the shunting space,” he said in an interview with Medical Design & Outsourcing. “Pressure sensing — which is not integrated into any current shunting technology that I’m aware of — we want to do that in a way that … can even push remote patient monitoring forward completely separate from the shunt space.”
Los Gatos, California-based Adona Medical (a Shifamed portfolio company) wants to enable heart pressure sensing at five or more times daily from inside both the arterial side and venous side for more informed treatment decisions such as medication dosing. Such a bi-atrial sensing system could even benefit patients who don’t need the shunt device.
“One of our longer-term goals is to actually create this monitor without a shunt in it,” Fahey said. “… Is [the shunt function] useful in some patients but less useful in others? The data will show us.”
An adjustable implant using shape memory nitinol

Adona Medical developed an electromagnetic catheter to adjust the size of its heart shunt device by heating shape memory nitinol. [Illustration courtesy of Adona Medical]
The second key technology would be the ability to adjust a catheter-delivered shunt device’s flow after it’s been implanted in a patient, which Fahey theorizes could improve outcomes for heart failure patients.
An expandable balloon catheter could make the shunt device larger to increase blood flow or even allow for interventional catheters to pass from the right heart into the left heart for ablation or other treatments.
To make the shunt device smaller, an electromagnetic catheter could deliver a burst of energy to take advantage of the nickel-titanium alloy nitinol’s ability to change shape when heat is applied.
Superelasticity in nitinol allows medical devices such as heart valves to spring back to their original shape after compression in a catheter for delivery. Nitinol’s shape memory, on the other hand, changes the shape of a nitinol device using temperatures that are too high for safe use if exposed directly to patient tissues.
During development, Adona Medical explored electrodes, hot balloons or high-temperature saline injections to trigger nitinol’s shape memory properties, but decided against them due to the potential harm on blood and tissue. Instead, the startup developed an electromagnetic catheter for non-contact heating of a subsection of the implant for less than five seconds.
“We can send some of that [electromagnetic energy to induce an] electrical current through a portion of our implant and resistively heat,” Fahey said. “The benefit there is that you’re heating from inside out, and you’re only heating the section that you want to heat. We’re not blasting in a huge bolus of hot fluid or we’re not using an electrode or a hot balloon that is inherently going to contact other things.”
Thermal insulation would prevent the heat from escaping and doing damage if the energy is precisely targeted and fast. Testing so far has shown negligible heating of blood and tissue, Fahey said.
“If you do your job right, what you’re left with is essentially a thermodynamics problem,” Fahey said. “Our initial testing suggests that this is possible.”
The entire device would be encased in expanded polytetrafluoroethylene (ePTFE) coating to encourage complete endothelialization within months to reduce the likelihood of thromboembolic events. The device will likely be MRI conditional.
Related: Medical nitinol manufacturing: How this nickel-titanium alloy is made for medical devices
Does shunt size matter?

Adona Medical co-founder and CEO Brian Fahey [Photo courtesy of Adona Medical]
There are many questions Adona Medical wants to answer as it develops this technology.
“Can some patients most optimally benefit from one amount of shunting versus another?” Fahey asked. “And if true, how does that evolve over time? … If you look at other medical therapies, we have determined that over time you have to adjust certain parameters — medication dosing or even some of these device therapy parameters — over time. To me, it seems crazy that the shunting therapy would be different.”
That question has yet to be answered because shunts don’t come in adjustable sizes. If they could be adjusted after implantation, pivotal clinical trials with heart failure patients could study safety and efficacy in delaying progression of the disease or alleviating symptoms. And an adjustable shunt device might expand the therapy to patients for whom a fixed-size device isn’t appropriate, but who might benefit from flow fine-tuning.
Adona Medical is designing its shunt device to have an adjustable flow ranging from approximately 5-10 mm. That would make it smaller than the smallest shunt currently being studied in humans at one end, and larger than the largest shunts at the other.
But the shunt could be designed to open as wide as 15 mm with a balloon catheter if a cardiologist needs to pass tools through, and then returned to its therapeutic flow size using nitinol’s shape memory.
“This is our design intent. We’ve shown it works in animal models, but have never done it in-human,” Fahey said.
What’s next for Adona Medical?
“The next big milestone is just getting to human use,” he said. “It’s on that variable timeline, but it’s in the line of sight.”
Fahey hopes to have the device in a live human for the first time within 12 months or sooner as the company hires to build out its team and raises funds from investors.
A key challenge that remains for Adona Medical is validating that its electromagnetic catheter can safely heat the nitinol shunt device to adjust blood flow.
“Our early testing indicates negligible temperature rises in the preclinical models during shunt size adjustment, but further validation is required,” Fahey said.
You can watch Fahey’s CSI Frankfurt presentation by searching for “Adona” here.