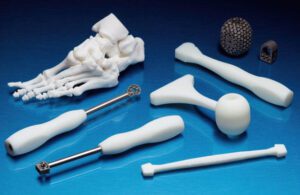
Restor3d uses metal and polymer 3D printing for orthopedic implants, anatomical models and surgical tools and guides. [Photo courtesy of Formlabs]
3D-printed orthopedic implant personalization and manufacturing abilities introduce new considerations for device developers.
3D-printed orthopedic implants are increasingly being used for patient personalization and features that improve osseointegration.
Faster, better and more affordable additive manufacturing technology is driving that adoption for ortho implant developers and manufacturers like Restor3d.
“The thing I’m most excited about is doubling down on this thesis that people deserve personalized implants — and that it’s actually possible now,” said Nathan Evans, SVP of product development at Restor3d. “Twenty years ago, it wasn’t possible. Ten years ago, people started doing it, but it wasn’t cost-effective. Today, it’s effective and able to be done in an extremely scalable and accessible way.”
Restor3d specializes in 3D-printed spine, foot and ankle, and upper extremity ortho implants, while Conformis — which Restor3d purchased in September 2023 — makes personalized knee and hip replacement implants.
In an interview with Medical Design & Outsourcing, Evans offered some basic know-how on 3D printing for orthopedic implants and how Restor3d and Conformis use additive manufacturing.
3D-printed orthopedic advantages
Restor3D and Conformis use a kind of 3D printing called selective laser sintering (SLS) to make custom implants personalized for individual patients based on CT scans. SLS printing uses lasers to build the implants layer by layer out of powdered metal such as titanium.
“Why should it be a one-size-fits-all business model where everybody gets an off-the-shelf knee?” Evans said. “… Every person is unique: their biomechanics, their body type, that deserves patient-specificity from an implant perspective, and the data does show, and science helps paint the story that when you do have a patient-specific implant, the outcomes are actually better and patients are more satisfied.”
SLS printing also allows for detailed surface geometry that encourages osseointegration, or bone growth into the implant for stable and secure fixation.
3D-printed, patient-specific instrumentation offers yet another advantage over non-personalized instrumentation in that it can better guide surgeons as they cut and drill. That reduces the need for assistance from surgical robotics systems, Evans said.
“They don’t need a robot’s aid in placement and cutting and prep, because the instruments now do it, whereas before, that wasn’t the case. The instruments were generic, and that’s why we actually needed assistance,” he said. “Our surgeons have given us that feedback, [saying] ‘I actually rely on navigation and robotics less than I did before because your instrumentation is so precise.”
3D-printed orthopedic challenges

Restor3d SVP of Product Development Nathan Evans [Photo courtesy of Restor3d]
While porous implant surfaces help with osseointegration, those surfaces also pose challenges.
“The more porous you make something, now you have deep inside of an implant a place for particulate to possibly get trapped, and you have new considerations with regards to things like cleaning,” Evans said. “How are you going to effectively validate your cleaning process to prove that you’re able to extract any contaminants and residuals? There’s upside for sure, but then you’ve got these interesting downsides.”
Another potential downside of porosity is that it could make a device weaker, so that adds another layer of complexity for engineers to test and design for.
Patient customization has drawbacks as well. Because the product specifications vary from patient to patient, they can’t be inspected for quality in the same way as a mass-produced implant.
“With traditional implants, it’s more linear dimensions. You can take a caliper and measure X, Y and Z,” Evans said. “But if you’ve got these highly organic, curved surfaces, how do you inspect that? It’s not easy.”
Restor3d inspects its customized implants with coordinate measuring machines, microscopes and profile comparators, among other traditional methods. A part can be 3D scanned, but it’s laborious, time-intensive and costly.
“The other challenge, interestingly enough, is a lot of those 3D scanners actually work best on matte surfaces,” Evans said. “A lot of our implants are extremely shiny and polished. The scanners do not work well on that. So then you’ve got to apply a coating to it [and then] take it back off.”
For now, that limits 3D scanning to R&D parts because Restor3d doesn’t have a validated method to remove that spray-on coating.
Materials for 3D printed ortho implants
Restor3d and Conformis use traditional manufacturing techniques for certain components that can’t be 3D printed or don’t need to be, like a total knee’s polyethylene bearing.
But most of what the companies make is 3D printed with titanium, cobalt chrome and — for certain accessories — resin-based polymers.
“Titanium in general is for fusion applications,” Evans said. “And cobalt is generally for arthroplasty, your highly polished bearing surfaces. … We print a ton of polymers as well in-house, not for implants but for ancillary instrumentation: things like trials, cut guides and impactors.”
Regulatory considerations and sterilization
Regulatory standards are a massive variable that can’t be overstated, Evans said. Because 3D printing for medical devices is still relatively new, the FDA is trying to keep up with the quickly evolving market.
“We have a very collaborative relationship with the FDA,” Evans said. “But it is a continuing challenge to make sure that the devices meet quality and regulatory standards and that there are appropriate standards for these devices.”
Restor3d ships most of its patient-specific implants non-sterile to hospitals, where they are sterilized with steam in autoclaves before they’re implanted. However, it uses gamma radiation to sterilize its OsseoRebar nails before shipping. Conformis sterilizes its patient-specific implants before shipping, primarily with ethylene oxide.
“Part of what we’re thinking through right now is kind of understanding how we envision providing these long term. I do think it’ll be sterile, but we’re looking at even novel sterilization methods [such as vaporized hydrogen peroxide] to make sure that can be done in a timely, cost-effective way.” Evans said.
Coatings for 3D printed ortho implants
Infection control is always a concern in orthopedic surgery, and porosity makes that even more challenging.
Antimicrobial coatings are among the technologies that Restor3d is constantly evaluating, along with other coatings and surface treatments that could, for example, further promote osseointegration.
“One of the challenges of porous structures — again, it’s the same thing with cleaning — if you’ve got porosity that is not line of sight, how do you make sure the coating effectively gets there?” Evans said. “A lot of the coating technologies are done via deposition, which is line of sight, so it can only hit what you’re spraying at. So, some of the newer methodologies are more like a dip where it would directly coat the whole thing, but this technology needs to evolve a little bit.”
While Restor3d is not currently commercially pursuing any coating technologies, “we’re a technology-first company, so we’re always going to be looking at things like that.”