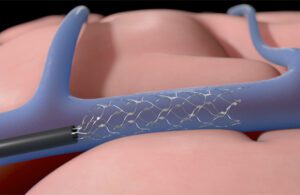
Synchron’s Stentrode device expands inside a blood vessel on the brain to relay motor signals [Illustration courtesy of Synchron]
Four ALS patients with a Synchron Stentrode brain implant had no serious adverse events one year after their procedure, which allowed the paralyzed patients to control a computer for online shopping, banking and text communication without using their hands or voice for input.
New York-based Synchron said the study — allowed by the FDA under an Investigational Device Exemption — demonstrated the safety of its brain-computer interface (BCI) technology. The device is delivered by catheter rather than the open-brain surgeries used by other neurotech developers like Elon Musk’s Neuralink.
Synchron uses the catheter to feed the Stentrode device through a patient’s vein to the blood vessels on the brain (the YouTube video below from 2021 shows the process). Then, the mesh-like device expands to line the vessel wall so its 16 electrodes can capture and relay movement signals from the brain’s motor cortex to another device in the chest that translates the signals into laptop computer commands.
The devices remained in place throughout the first year of the study and the blood vessel remained open, Synchron said ahead of a presentation at next month’s American Academy of Neurology conference. The study will continue in the U.S. and Australia as it opens to more patients.
Synchron said it is the only company with FDA approval to conduct clinical trials of a permanently implanted BCI. The company said it achieved the first human implantation in 2019 and was granted breakthrough device designation by the FDA in 2020.

Synchron co-founder and CEO Dr. Thomas Oxley [Photo courtesy of Synchron]
“These results are a huge advancement for the field of BCI. We’ve shown that our endovascular BCI approach is safe, and allows patients to accomplish daily online tasks without invasive brain surgery,” Synchron co-founder and CEO Dr. Thomas Oxley said in a news release. “Years of research and technological development culminated in this moment, and as we continue to develop our technology, it has the potential to change the lives of millions of patients globally who suffer from paralysis and other debilitating neurological and brain-related diseases.”
The company conducted the study with support from the U.S. Defense Advanced Research Projects Agency (DARPA), the Office of Naval Research, the National Health and Medical Research Council of Australia, the Australian Federal Government Foundation and the Motor Neurone Disease Research Institute of Australia.
New York-based Synchron has offices in California’s Silicon Valley and R&D facilities in Melbourne, Australia.