
This close-up shows one of the electrodes on Synchron’s Stentrode brain implant. [Image courtesy of Synchron]
Synchron’s efforts to develop, manufacture and commercialize its groundbreaking brain-computer interface system holds lessons for device developers who are designing increasingly miniaturized implants for minimally invasive delivery.
In recent months and years, we’ve asked Synchron leaders to share their advice as they studied the safety of their nitinol Stentrode implant, refined the implant’s design and manufacturing process, and launched a clinical trial under an investigational device exemption from the FDA, which previously awarded Synchron with breakthrough device designation.
‘Take it step by step’
In a wide-ranging interview with Medical Design & Outsourcing, Synchron CEO and co-founder Dr. Tom Oxley offered some advice specifically for device designers and engineers.
“You have to take it step by step,” Oxley said. “… Engineering perfection will stop you from getting to market quickly [and] learning. Maybe engineers don’t like and trust physicians that much, but give physicians a chance.”
Here’s one example: the advent of flow-diverting stents, with Medtronic’s Pipeline embolization device beating Stryker’s Surpass to market.
Stryker took longer “because they wanted to get the stiffness right. Pipeline came out really quick, but they had a very stiff device,” Oxley said. “You know what? The physicians could handle it. And the mechanical problem that hadn’t been solved pushed over to the physicians in the field, and they solved it.”
Design for manufacturing

A close-up of the Synchron Stentrode brain implant [Image courtesy of Synchron]
Synchron Chief Technology Officer Riki Banerjee offered some advice of her own, noting that she’s “always gravitated toward these very complex interdisciplinary spaces” and understanding the intersection of different disciplines, starting with user needs, through use conditions and all the way through manufacturing.
“Any mechanical implant, understanding those use conditions, which are: How is the physician handling it? How is it implanted in the body? How does it stay in place in the body? What are the chronic effects in the body? And really understanding that. And then the other side of it is, OK, so you can take those inputs and come up with designs, but the DFM (design for manufacturing) side of it — how do you make sure that design is manufacturable? Because if you end up in a lot of novel process development, that will inhibit your ability to scale and build product, too. Or a very novel supply chain, for example, could be very, very, very challenging.”
Synchron implant challenges
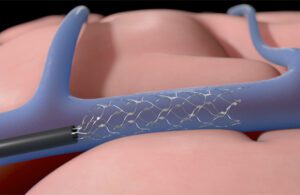
Synchron’s Stentrode device expands inside a blood vessel on the brain to relay motor signals. [Illustration courtesy of Synchron]
Synchron Senior Director of Neuroscience Peter Yoo explained the advantages of using a catheter to place the Stentrode implant inside a patient’s brain, rather than open-brain surgery.
“The novel approach of the catheter delivery increases the number of physicians who can deliver our devices compared to a very specialized type of surgery,” Yoo said in an interview. “The techniques that we use are standard angiography procedures and other neurointerventional techniques performed by stroke doctors. We’re hoping that it’ll help with the proliferation of the technology and access to the technology, that it can be cheaper and more available for the patients who are in need.”
He also offered his perspective on the obstacles the company faced while developing the technology.
“The main challenge was there was essentially a lack of chronic data of leaving a device inside the blood vessel permanently,” he said. “There is one routine case now: treating intracranial idiopathic hypertension [where] for an unknown reason, intracranial pressure builds up. People have been putting a stent into the transverse sinus — which is the sinus that runs along back of the head sideways — permanently placing stents there with less than a 2% major complication rate. But that’s the only chronic-stenting-inside-the-brain data that’s available. So we had to do a lot of work in the background, benchtop testing and some large-animal testing to make sure that leaving a permanent device with the lead inside the blood vessel doesn’t cause thrombosis or any other health risks.”
A major engineering challenge was creating many of the manufacturing processes used to make the Stentrode implant, Yoo said.
“Mounting electronics onto stents that are going to be permanently implanted didn’t exist prior to us. We started off manually making them, now we’re printing them,” he said. “All of those processes and methods had to be created from scratch, leaning on a lot of other manufacturing practices and know-how. And we had to solve the problem of once you make those recording heads, the sensors, then how do we connect those into blood vessels and connect it into some transmitting units. All of those transition zones and coming up with electronics to wirelessly transmit the data out of the body was also a problem and a challenge. The tortuosity of the blood vessels means that the device had to be flexible, yet firm enough so that you can push the device into the brain through those tortuosities. Getting those sweet spots right was definitely a challenge for our mechanical team.”
Commercial strategy — and focus
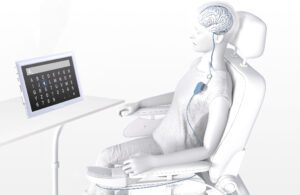
Synchron’s brain-computer interface (BCI) system uses the Stentrode brain implant to sense brain signals and relay them to a receiver implant in a patient’s chest. [Illustration courtesy of Synchron]
“There’s no quick path to get to market with an innovation like this,” Synchron Chief Commercial Officer Kurt Haggstrom said in a DeviceTalks interview. “It has to go through the traditional Class III medical device route we all know from an implantable standpoint … all the same safety regulations, performance regulations. We’re going to take that path and partner with the FDA to take this to market.”
Simplicity is key for commercializing an innovation like this, he said.
“Out of the box, calibrating these things, it has to be really usable for patients. And that’s really where we’re centered around,” he said.
Until then, a critical challenge will be remaining selective about how to apply the startup’s limited resources, prioritizing patient needs to develop a system that addresses core patient needs.
“It is all about focus. … In this space, because of the novelty and how the brain works and all the signals that you can read, you can easily get distracted doing different activities [like] controlling robotic arms and all these things,” Haggstrom said. “But ultimately, what they need is a tool they can use every day to be able to reconnect to the world. So for me, the race is keeping our team focused on our spend.”