
SeaStar Medical’s Selective Cytopheretic Device (SCD) [Image courtesy of SeaStar Medical]
SeaStar Medical recently won FDA breakthrough device designation for a new way to treat hyperinflammation with technology that could help fight chronic conditions from COVID-19 infections.
The Denver-based medical developer is seeking a humanitarian device exemption for treating children with acute kidney injury (AKI) based on an FDA-funded study, SeaStar President and CEO Eric Schlorff said in an interview with Medical Design & Outsourcing.
The company is also planning a pivotal trial with around 200 adult AKI patients in early 2023, focused on reducing mortality and dialysis dependency.

SeaStar Medical CEO Eric Schlorff [Photo courtesy of SeaStar Medical]
SeaStar most recently studied the use of its Selective Cytopheretic Device (SCD) on 22 COVID-19 patients with multiple organ failure in ICUs. The treatment reduced mortality to 50% (compared to 81% in the control group), while patients who used the device for more than 96 hours saw an even lower mortality rate of 31%.
The company used COVID-19 complications to demonstrate how safely and effectively the SCD is in treating hyperinflamation across different organs regardless of the cause, whether it be viral, bacterial or from trauma or surgery.
“We address the cytokine storm or hyperinflammation,” Schlorff said. “We are not going to reduce the front end, the pathogen or whatever it is. We look at this as insult agnostic, so we don’t really care how it starts. What we care about is how we can actually take that patient from a pro-inflammatory state to a reparative state.”
SeaStar’s device works as an add-on to continuous renal replacement therapy, which is 24-hour dialysis for ICU patients who can’t handle the faster flow rates of traditional hemodialysis. The SCD doesn’t filter out immune cells, but instead calms the most active damaging ones without affecting the reparative cells, Schlorff said.
“We are able to collect the most highly activated immune cells,” he said. “We sequester them and turn the volume down. I like the analogy of a radio. A steroid will turn the radio off, but you want to listen to the radio, so why not just turn the volume down? In our case, we’re turning the volume down in the most highly activated of these molecules or these effector cells that are causing all this damage. So if you can turn that down, you allow that body to actually repair.”
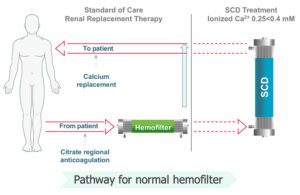
SeaStar Medical’s SCD treats a patient’s blood after it’s been filtered by a separate continuous renal replacement therapy device. [Image courtesy of SeaStar Medical]
The SCD’s trick is mimicing the flow rate of the body’s capillary beds to which immune cells adhere, then calming the most highly activated cells before sending them back into the body. SeaStar is focused on AKI for now, but Schlorff said the technology’s ability to reduce hyperinflammation means it will likely also fight acute respiratory distress syndrome in the lungs, myocarditis in the heart and other inflammatory conditions throughout the body.
“We potentially have a tool that can go across any of these different organs,” Schlorff said. “We don’t have to think about things as a heart issue or a kidney issue. No, it’s an inflammation issue. And obviously, chronic inflammation is a major concern for all of our long-term health.”
The SCD could one day be used outside of the ICU for hospitalized patients, potentially reducing the risk of long-term organ damage from COVID-19, for example.
“There is a lot of AKI that’s actually occurring well after hospitalization [for COVID patients],” Schlorff said. “This is definitely a concern for all of these patients, just because they’re out of the hospital doesn’t mean that there’s not long-term implications. And I think that’s what we’re going see with the heart and the lungs. I think this is a long-term thing. We just don’t have the data today because we haven’t been in it long enough.”
SeaStar estimates a multibillion-dollar market in the U.S. for AKI, not counting the potential for expansion into ARDS, extracorporeal membrane oxygenation (ECMO) and other indications.
Dr. Kevin Chung is set to start as SeaStar’s chief medical officer on July 1.
The company is planning to go public in the third quarter of 2022 through a combination with special purpose acquisition company LMF Acquisition Opportunities (Nasdaq: LMAO).