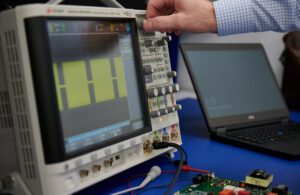
An engineer develops the pulsed waveform output for a PFA system. [Photo courtesy of Minnetronix Medical]
An engineer experienced with radiofrequency (RF) ablation systems might wonder what all the fuss is about when looking at the design of a pulsed field ablation (PFA) system.
Compared to an RF ablation generator, a PFA generator is going to look much less complicated, perhaps with only a tenth as many parts. But the seemingly simpler schematics can be deceiving.
“The difference is that in a PFA power supply, the way that those components are being used is just so different, and they’re being stressed to the limits of what their ratings are in a way that just isn’t obvious to most engineers,” said Dan Friedrichs, an engineer at Minnetronix Medical. “… There’s more complexity than meets the eye.”
Friedrichs leads development engineering efforts at Minnetronix Medical for the commercialization of surgical energy devices. He’s also shared his expertise with Medical Design & Outsourcing readers through several contributions on the topic.
As the first PFA systems win FDA approval for treating atrial fibrillation (AFib), we asked Friedrichs and his colleague, Aaron McCabe (who, as Minnetronix’s technology segment director, focuses on surgical energy applications), to explain some of the design considerations for PFA generators. These generators send energy pulses through catheters inside the heart to disrupt erratic cardiac cells.
“Some of the specs for these PFA systems require that you pick just these enormous transistors, even though the transistors aren’t processing a lot of power — they don’t actually get hot,” Friedrichs said. “… You open the lid of one of these power supplies and see these giant transistors, but they aren’t mounted to heat sinks. They aren’t processing any large amount of power, but those are the types of parts that you have to select to meet what turns out to be very esoteric requirements around delivering the types of pulses that are necessary in PFA.”
“And that extends basically to all the components. Something like a capacitor inside a PFA generator that has these really strong pulsed currents that are uncommon in most other power supplies, you have to select just the right type of capacitor for it,” Friedrichs continued. “Every electronic component that goes into the power supply superficially looks very easy, but to actually get it to work, there’s all sorts of tricks that are not obvious.”
PFA generators and catheters create energy fields that can open temporary or permanent holes in the walls of targeted heart cells through a process called electroporation without heat or damage to nearby nerves. A PFA procedure for AFib can be completed hours faster than the same procedure with RF, while it has less risk of damaging surrounding tissue because it’s nonthermal.
“If you were to look at the outputs of these PFA systems, a typical specification could be 2,000 volts and 100 or 200 amps,” Friedrichs said. “You’d say, ‘Oh my gosh, that’s 200 kilowatts of power. Are we going to vaporize the patient when we connect this to them?’ But it turns out that the duty cycle is very short, like 1% or 2% on time, so the average power delivered to the patient is a watt or two at most. It’s not a thermal effect. But to design a power supply that’s capable of supporting those instantaneous high voltages and those instantaneous high currents. That’s actually a technical challenge.”
“Because of those really high instantaneous voltages and currents, there is consideration for how do you, for example, route cables within a box, because high currents flowing through cables create magnetic fields, they interact with other components,” Friedrichs continued. “So the tolerances and the design controls that you need to go through to ensure that you get a consistent repeatable result are significant and probably not obvious.”
The right recipe for PFA
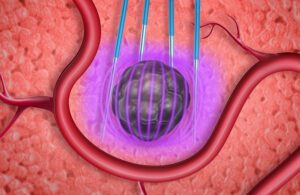
Electroporation uses an external electrical device to apply a prescribed electric field to tissue. This can temporarily open pores in cell walls, allowing transport of drugs, DNA, and gene therapies — or permanently open them to kill targeted heart tissue or cancer cells. [Illustration courtesy of Minnetronix]
With RF ablation, there’s generally only one variable to control when a thermal catheter is in the heart: time.
“We don’t want to create steam in the heart, so we set the temperature just below 100°C, just below boiling. Then it’s just a matter of time, because time and temperature together is what damages tissue. But that’s also the reason why these are long procedures,” Friedrichs said.
Time is just one variable with PFA, and the pulses are generally measured in microseconds or milliseconds.
“With PFA we have literally an infinite number of permutations of the variables,” Friedrichs said. “We have the pulse amplitude, we have the pulse width, we have the spacing between the pulses, we have the number of pulses, we have the polarity of the pulses, often there will just be arbitrary delays between different pulses, there’s recipes that have different amplitudes of pulses. Those are the parameters that vary — and that’s just on the generator, and then we connect it to a catheter and the catheter’s geometry can vary as well. We have not only an infinite space of variables that can be tuned to this, but then it’s also really hard to measure which of those given recipes is effective.”
While the voltage is generally thousands of volts, the more important measure is volts per centimeter: field strength, which can be modified by changing the spacing between electrodes on the catheter. The amplitude of the pulses is “probably the strongest lever in getting the tissue selectivity that we want,” Friedrichs said, referring to PFA’s ability to ablate only heart muscle cells while sparing nearby nerves in the esophagus.
“But then also it’s the width of the pulse,” he continued. “We’re applying a pulse that has an amplitude and a duration. We have a delay time before we deliver the next pulse in the sequence, and that can be different than the pulse width. Then there’s the number of pulses we apply. The pulses don’t all have to be the same polarity — for instance, we can have positive pulses, then we can have a negative pulse, or we could have two positive pulses and then a lower amplitude but longer negative pulse. And so all those combinations become an infinite number of permutations.”
The timing of pulses can help counteract muscle stimulation, one of PFA’s potential side effects. That could trigger a heart contraction (or systole) or cause a patient to cough, which in turn could cause trauma if they’re on a ventilator.
“The difference is between a heart defibrillator and one of these systems [is] timing,” Friedrichs said. “Can we rely on software to get the timing correct 100% of the time, or do we think that there’s a one in a million chance that the software might crash while we’re delivering the therapy to this patient?”
To avoid that risk of a crash, a device developer might instead write code on a field programmable gate array (FPGA) or implement the control in purely discrete analog circuitry.
“Good partitions between software and hardware enable confidence and safety,” McCabe said.
And while PFA catheters are usually larger than RF ablation catheters — meaning more risk for accidental mechanical damage within the heart — PFA’s nonthermal and tissue-selective therapy is a big step forward in safety.
“Even if the only thing that PFA gives us is safety against accidentally burning the esophagus — which is, to begin with a rare situation, maybe a one-in-1000 type of situation — even that is valuable enough to make this wholesale change in this multi-billion-dollar market,” Friedrichs said.
Designing PFA systems for flexibility

A Minnetronix engineer evaluates a control board for an electroporation system. [Photo courtesy of Minnetronix]
“We have so many variables, and we don’t have a good understanding of which variables are important,” Friedrichs said. “… When you compare it to legacy technologies like RF ablation, RF ablation has a lot less concern about process control in production of equipment, because we can actually measure what’s happening in the tissue. We literally have a temperature sensor in the tissue and we can measure that it’s being effective, and the variation back in the generator, it almost doesn’t matter. But with PFA, we don’t have the ability to detect that in the tissue. And then we also don’t have the ability to assess efficacy in real-time. So we’re really relying entirely on the recipe of how the pulse generator was built to be consistent and to give the same thing for every patient.”
In the design phase, that requires flexibility to dial in the appropriate dose titration for effective therapy, said McCabe.
“Unless you’re fortunate enough to work at one of the places that has already cracked it and have their own proprietary methods for treating, more than likely you’re at the beginning of that journey and you want to be able to dial in and alter the different parameters of your pulse,” McCabe said. “You’ve got kind of two sides of the same coin here: you need a lot of flexibility, and then you need to transition to be able to also lock down consistently and repeatedly once you’ve nailed it.”
Because there aren’t a lot of electronics operating with similar parameters as PFA generators, device designers may need to consider customized components.
“One of the challenges we face on all surgical energy systems is that we have to electrically isolate the patient from mains power for safety,” Friedrichs said. “Components like transformers, digital isolators and isolated power supplies don’t exist specifically for this type of system. That requires us to be very creative and either design our own or adapt something on the market to fit these unique requirements.”
The future of PFA
While PFA in its current form is generating excitement for its speed and safety, one issue on safety regulators’ radar is bubble formation inside the heart during treatment. Bubbles — like those created by boiling blood above 100°C with thermal ablation — create a risk of embolism, and PFA system developers such as Abbott have discussed the risks with the FDA.
“There’s other mechanisms by which you could have bubbles,” Friedrichs said. “You’ve got voltage in a ionic solution — you can create electrolysis, separating ions and create chemical reaction products that way. It’s also possible to have very, very localized heating because these electrodes may have sharp edges, and so you could have hotspots there. I think the science is unsettled as to how significant of an issue this could be. But it’s unavoidable.”
Friedrichs said he’s interested to see how PFA will develop in the years ahead. While thermal fields are very difficult to model computationally, the equations for electric fields are simple.
“You can imagine a future where we are able to analytically plan out what kind of energy dosage we want to give a patient because right now, it’s more or less, ‘You have this disease, you are getting this dose.’ It’s not patient specific, it’s not titrated to the extent of their disease,” Friedrichs said. “But in the future, you can imagine that we would be able to actually come up with a patient-specific energy prescription and deliver it just for that patient’s disease. And that’s unique to electric fields.”