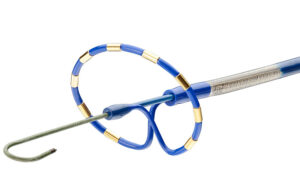
The Medtronic PulseSelect Pulsed Field Ablation (PFA) System is designed to treat paroxysmal and persistent atrial fibrillation (AFIb). [Image courtesy of Medtronic]
Medtronic’s PulseSelect Pulsed Field Ablation (PFA) System — which won the first FDA approval for PFA to treat atrial fibrillation (AFib) — is just the start of a wave of new PFA devices expected to hit the market.
Medtronic is lining up another PFA cardiac ablation system for approval, while competitor Boston Scientific anticipates approval of its Farapulse PFA system sometime in 2024. Meanwhile, Johnson & Johnson’s Biosense Webster is testing its ThermoCool SmartTouch SF system for both PFA and radiofrequency ablation.
Medtronic EVP and Cardiovascular President Sean Salmon recently discussed PFA technology in an interview with Medical Design & Outsourcing before the world’s largest medical device manufacturer announced the PulseSelect approval.
“The whole promise of pulsed field is it can be faster, safer, and at least as efficacious as what you can do with thermal ablation today,” he said.
An estimated 60 million people are affected by AFib, which is a progressive disease that can increase the risk of heart failure, stroke and death. The FDA designated Medtronic’s PFA systems (as well as Boston Scientific’s Farapulse) as breakthrough devices for the potential to more effectively treat serious conditions like AFib.
“There’s a huge opportunity because it’s a $9 billion market that’s way underpenetrated — there’s about 5% penetration — and it’s growing double digits right now,” Salmon said. “We have a great opportunity to enter that market with highly differentiated technologies that can have a lot of growth for us for many years to come, and of course, help a lot of patients.”
Pulsed field ablation, Salmon explained, “uses a field of electricity, these pulse trains that basically rupture a cell membrane, water comes in there, then you have this death by apoptosis of the cell. It allows you to really have a very rapid and incredibly safe procedure.”
Medtronic’s PulseSelect system can be used by itself, with mapping or navigation platforms manufactured by other firms, or used with Medtronic’s Affera technology.

Affera’s Sphere-9 mapping and ablation catheter can provide both radiofrequency ablation and PFA to treat AFib. [Photo courtesy of Affera]
Affera’s system combines high-density mapping, navigation and ablation. Affera’s Sphere-9 catheter is a point-by-point ablation catheter with the ability to delivery both radiofrequency and pulsed field energy, with a foot pedal allowing the cardiologist to control the energy delivery.
Related: Why Affera’s cardiac ablation technology is worth $1B to Medtronic
“What’s really unique about that is the same catheter can go in there, create a beautiful high-density map that tells you what’s going on in the heart, and then that same catheter can be used to deliver either pulse field or radiofrequency ablation,” Salmon said. “… It gives a really nice view of [whether] you put your lines of conduction in the right place.”
The Affera mapping system will be backward-compatible with Medtronic’s cryoablation technologies, PulseSelect and more traditional radiofrequency technologies.
PulseSelect is a single-shot ablation technology (like cryotherapy), which only represents about 15% of the cardiac ablation market. The more dominant method is point-by-point ablation, with Salmon estimating it represents 85% of cardiac ablation procedure volume.

Medtronic EVP and Cardiovascular President Sean Salmon [Image courtesy of Medtronic]
Salmon said Medtronic finished enrollment on its Affera system for treating persistent AFib about a year ago and expects to read out one-year results in or around spring 2024. At the same time, Medtronic will file the data with the FDA in its regulatory submission for approval. FDA approval could come as early as that year.
“PulseSelect has been proven already in the two populations that matter the most — paroxysmal as well as persistent [AFib], the more difficult one — and performed well on both of those,” Salmon said.
PFA technology also has a connection to renal denervation (RDN). Medtronic bought Ardien in 2011 and won FDA approval for its radiofrequency energy Symplicity Spyral RDN system in November 2023.
PFA was one of the technologies that Ardien tried to use to treat hypertension with RDN, which destroys overactive nerves in the renal arteries to lower blood pressure. But PFA didn’t work for RDN because it only kills muscle tissue, not nerves, Salmon said.
“It almost undid Ardien until they moved to radiofrequency energies,” he said.
Because PFA doesn’t kill nerves, it’s a better technology for cardiac ablation to avoid damaging the phrenic nerves and nerves in the esophagus, Salmon said.
PulseSelect “handily met our safety and efficacy endpoints with really great clinical efficacy, great and quality improvement in life,” he said.
Related: What Medtronic learned on its long road to RDN approval