 Eli Lilly‘s (NYSE:LLY) tirzepatide achieved up to 15.7% weight loss in the SURMOUNT-2 study, sparking a potential tirzepatide vs semaglutide competition in the obesity and type 2 diabetes treatment markets. The phase 3 study enrolled 938 participants with diverse backgrounds.
Eli Lilly‘s (NYSE:LLY) tirzepatide achieved up to 15.7% weight loss in the SURMOUNT-2 study, sparking a potential tirzepatide vs semaglutide competition in the obesity and type 2 diabetes treatment markets. The phase 3 study enrolled 938 participants with diverse backgrounds.
Tirzepatide promises to be a megablockbuster with a number of analysts pegging peak annual sales hitting $25 billion. Few drugs have surpassed the $20 billion threshold. One notable example is AbbVie’s monoclonal antibody Humira (adalimumab), which hit $21.2 billion in sales in 2022 and $20.7 billion a year earlier. The drug was the world’s bestseller until the COVID-19 pandemic stoked demand for the Pfizer-BioNTech vaccine, whose cumulative annual sales hit $59.1 billion in 2021.
Lilly has signaled its plans to submit tirzepatide to the FDA, potentially leading to competition between tirzepatide and semaglutide in the obesity and type 2 diabetes treatment markets.
Tirzepatide’s potential to disrupt the obesity treatment landscape
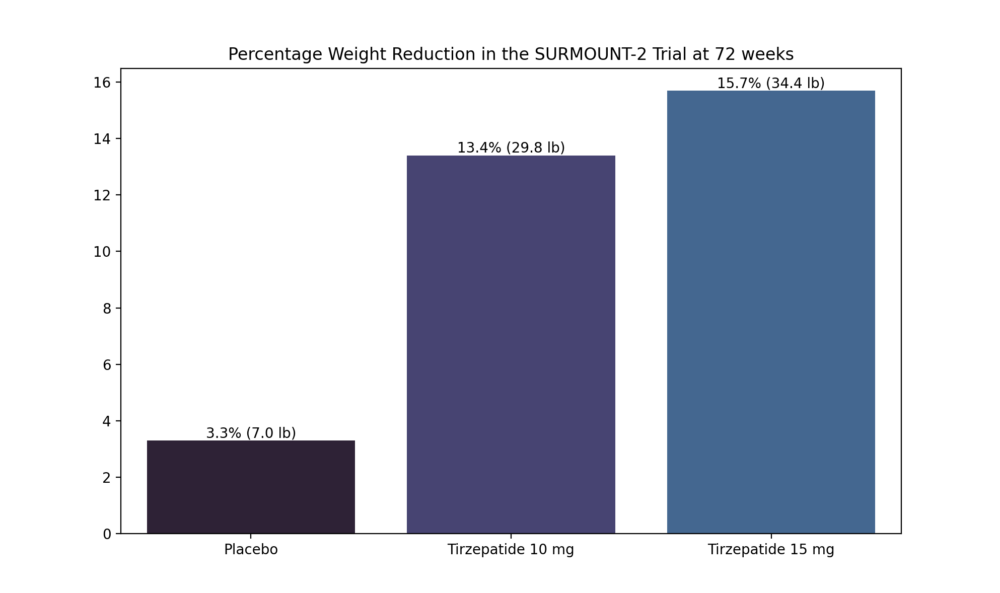
SURMOUNT-2 weight loss compared to placebo.
Semaglutide, under the brand name Wegovy, has been on the market after FDA approved it in 2021 for some adults who are obese or overweight. Wegovy is administered as a once-weekly subcutaneous (under the skin) self-injection with a typical dose of 2.4 mg for weight loss. Clinical trial results showed that participants experienced 15% to 18% weight loss during the trials.
In the SURMOUNT-1 trial, tirzepatide demonstrated substantial weight loss of 16.5% to 22.4% over 72 weeks in people without diabetes. The study enrolled 2,539 non-diabetic participants who were obese or overweight with at least one weight-related condition.
Tirzepatide is the first dual GIP/GLP-1 receptor co-agonist to win FDA approval for type 2 diabetes. The drug activates key mediators of insulin secretion that also regulate food intake. Tirzepatide thus offers “unmatched effectiveness” in terms of glycemic control and body weight reduction, as a report in Cardiovascular Diabetology noted.
The cost-effectiveness of tirzepatide vs. semaglutide in obesity
An analysis in Diabetes, Obesity and Metabolism suggest that the cost per 1% of body weight reduction is lower for tirzepatide than semaglutide. This article pegs tirzepatide’s cost per 1% body weight reduction at $1,196.90, while it estimated semaglutide’s cost for the same benchmark at $1,511.17. The overall cost of therapy with tirzepatide topped $17,500, while the cost for semaglutide was almost $23,000. Although there have been no head-to-head trials yet, tirzepatide could potentially lead to more significant weight loss with a comparable safety profile to semaglutide, according to an article in Frontiners in Endocrinology.