SmartCube integrates behavioral neurobiology, robotics and computer vision to process and analyze large temporal and vector-based datasets. This platform uses proprietary bioinformatics and probabilistic causal inference algorithms to explore compounds’ potential to treat psychiatric disorders. [Image courtesy of PsychoGenics]
In an era of rapid AI progress, the quest to pioneer the first AI-developed drug candidates has led to an increasing number of these drug candidates entering clinical trials. One contender is ulotaront, an antipsychotic drug, that fared well in a phase 3 schizophrenia study published in NEJM in 2020. The trace amine-associated receptor 1 agonist has entered phase 2/3 studies to test its potential in generalized anxiety disorder and as an adjunctive therapy for major depressive disorder treatment.
“Not only is this the first drug discovered using machine learning that’s this close to market, but also a proof of the potential of a polypharmacology approach for complex diseases like central nervous system (CNS) disorders,” said Daniela Brunner, the chief innovation officer of PsychoGenics.
Moving beyond the AI hype
Despite the surrounding hype, AI projects in drug development are rapidly maturing, becoming “extremely interesting” when applied to specific challenges, as Brunner pointed out.
PsychoGenics is exploring the use of machine learning across multiple domains. For starters, it aids in designing their compound libraries. “With billions of purchasable compounds available, we need to select those which will be most beneficial for our in vivo studies,” Brunner said. “Machine learning helps us narrow down these choices, selecting compounds that fall within certain parameters based on their expected activity in the SmartCube system.”
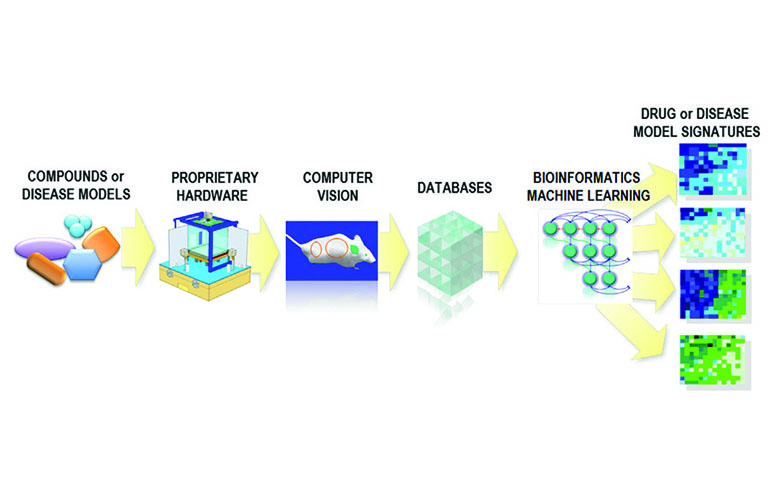
SmartCube is a platform PsychoGenics developed for phenotypic drug discovery for CNS disorders. It provides an unbiased, mechanism-agnostic method to screen compound libraries. The platform, combining custom hardware with an automated testing sequence, presents challenges to a mouse to capture more than half a million data points per mouse per session. The system compares the resulting massive dataset to a proprietary reference database to gauge the potential of compounds to treat psychiatric disorders at the drug class and subclass level.
The Star Trek inspiration behind SmartCube

Daniela Brunner, Ph.D.
Star Trek partially inspired SmartCube, Brunner shared humorously. “We were thinking, if we could follow the mouse around and see everything that mouse does, we will be able to tell whether the mouse we just injected got an antidepressant or an anxiolytic,” she said.
In Star Trek, the cast used futuristic sensors and scanning devices to gain information about unknown objects or lifeforms. Similarly, PsychoGenics aimed to use sensors to characterize every single movement of rodents.
This led to the concept of SmartCube, which Brunner describes as a robot. “In essence, we’ve created a robot where the mouse is placed inside,” she said. “Our team of robotic engineers crafted this system to meticulously track the mouse’s every movement, capturing an immense amount of data,” she added. “By putting the animal inside, we can track everything.”
Labeling drugs: Antidepressants, anxiolytics and beyond
A key question that arose was about the data to be collected. Brunner explained, “We targeted labeled data, drawn specifically from effective human antidepressants. This label, derived from human trials, characterizes the unique behavioral signatures observed in the mouse.”
She emphasized the perspective toward studying the mice. “We don’t pretend that the mice are depressed or schizophrenic,” Brunner said. “We’re emphasizing that a mouse is a complex mammal. When a drug interacts with its diverse array of receptors, it creates a unique behavioral pattern, which we refer to as, say, an antidepressant signature.”
The process results in a kind of back-translation from humans to mice. “Our labels originate from human trials, while the data is collected from mice. We hope that this can be forward-translated when we test another drug,” Brunner said.
When using the SmartCube, the company has the option to select either commercially available compounds or generate completely new molecules based on what it has learned from SmartCube experiments.
PsychoGenics uses machine learning within the SmartCube to convert video footage of mice into numerical data for analysis.
Computer vision, a subset of machine learning, assists in the conversion. Brunner highlighted the improvements in computer vision stemming from deep learning, enabling it to identify specific mouse behaviors. “Computer vision has been around for more than 30 years,” Brunner said. “It is much, much more powerful than what it was before.” Whereas in the past, a simple computer vision system might track a mouse’s movement, current computer vision systems can identify specific mouse behaviors.
Additionally, PsychoGenics employs another layer of machine learning to train algorithms for recognizing specific behavioral signatures related to different drug types. “Machine learning plays a crucial role at every stage of our process — from selecting and creating compounds, to interpreting and analyzing the data,” Brunner said.
The SmartCube loop and continuous refinement in drug development
The SmartCube system facilitates a seamless transition from data to drugs. It comprises an iterative loop of drug testing, data collection, machine learning analysis and drug optimization. “This process continually refines the drug candidates, guided by clear behavioral signatures, and continually validated in in vivo conditions,” Brunner said.
In addition to SmartCube, PsychoGenics has developed a computer vision system to spot changes in gait geometry known as NeuroCube. A system known as PhenoCube provides an environment to evaluate disease models or treatments over an extended period. Finally, PsychoGenics’ eCube system is an EEG-based discovery platform for exploring the effects of compounds from various drug classes on electrical brain activity.
Brunner sees the role of such systems as a harbinger of the future. Referring to SmartCube’s role in developing ulotaront as an example, Brunner predicts, “We’re about to witness not just the launch of the first drug developed with machine learning, but also a demonstration of the effectiveness of a polypharmacology strategy in treating complex central nervous system disorders.”