Orthofix Medical (Nasdaq:OFIX) announced today that it surpassed 5,000 device implants for its Fitbone TAA intramedullary limb-lengthening system.
Lewisville, Texas-based Orthofix said the system features more than 20 years of clinical history. Over that time, the device demonstrated safety and effectiveness in limb lengthening and deformity correction in adults and children.
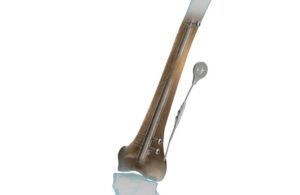
Orthofix acquired the Fitbone intramedullary lengthening nail from Wittenstein SE in March 2020. The fully implantable system corrects leg length and deformity discrepancies through a minimally invasive implantation procedure.
Fitbone features a motorized intramedullary nail, a subcutaneously placed receiver and an external control set. This enables the patient to manage the distraction phase at home. Once the patient completes their treatment, they have the nail and receiver removed.
Orthofix offers Fitbone in the U.S. through FDA 510(k) clearance. The company said it marks the only lengthening nail with a pediatric indication. It also offers Fitbone in Europe through CE mark approval.
“Clinical experience and published, peer-reviewed data for the Fitbone intramedullary limb-lengthening system continue to reinforce the safety and efficacy of this life-changing technology,” said Kim Elting, Orthofix president of global orthopedics. “We are the only company to offer a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction and deformity correction, and Fitbone is a key technology platform we intend to continue to build on to offer surgeons the best internal solutions for their patients.”