Continuous glucose monitors (CGMs) have transformed how many people with diabetes manage blood sugar, but attempts to monitor blood glucose have a long history.
Attempts to manage glucose kicked off in earnest when researchers began measuring glucose in urine in the mid-1800s. Scientists’ ability to do so steadily improved over the years, but urine glucose testing wasn’t commercialized until 1908, establishing a foundation for diabetes care.
Elkhart, Ind.-based Ames Company refined the process in 1945 with the introduction of Clinitest reagent tablets, which are still commercially available, albeit from Bayer (ETR: BAYN). The company would introduce the first blood glucose test strip in 1965. The Dextrostix-branded strips were intended for use in doctors’ offices.
In the 1970s, Ames developed a device known as the Ames Reflectance Meter to measure reflected light from a Dextrostix strip. It was the first blood glucose meter and was intended for use in doctors’ offices.
Home blood glucose monitoring became feasible in the early 1980s with the launch of the Ames Dextrometer. This electronic glucometer allowed people with diabetes to check blood glucose in about one minute. More affordable blood glucose monitors would follow that require less blood than the Dextrometer.
While self-monitoring blood glucose technology continued to improve from the 1980s to the early 2000s, patient continuous glucose monitors didn’t become commonplace until the new millennium.
1999: FDA approves first professional CGM
Known as a “Continuous Glucose Monitor System” from Medtronic MiniMed (Medtronic Diabetes, Northridge, Calif.), the device provided three days of glucose data meant initially for a physician rather than a patient to review. The device was intended to help physicians establish patients’ glucose profiles and monitor insulin therapy. It took patient readings every 10 seconds while providing 5-minute averages. The sensor had a maximum life of 72 hours.
Like many others that would follow, the device would require fingerstick-based blood glucose calibration.
2001: FDA approves first real-time CGM
The GlucoWatch Biographer from Cygnus Inc. won marketing approval for adults with diabetes. It was the only system to provide a stream of glucose readings to patients when introduced.
While the armband-based device was noninvasive, it was not widely successful because it tended to cause site irritation.
Animas, which eventually acquired the rights to the GlucoWatch and its successor, the GlucoWatch G2 Biographer, stopped selling the technology in 2007. Johnson & Johnson acquired Animas in 2006.
2004: Medtronic wins FDA approval for its Guardian CGM System
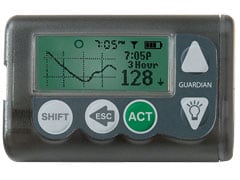
The Guardian CGM from the medical device giant could warn users when their blood sugar hits dangerous levels. The device provided glucose values every five minutes.
2004: Dexcom introduces its first real-time CGM
Known as the Dexcom STS (short for short-term sensor), the device shared glucose every five minutes. Like Medtronic’s Guardian device, the STS could also provide alerts when glucose levels dip or rise above predefined thresholds. The device was intended to be used for up to 72 hours.
2006: Medtronic debuts first integrated insulin pump and CGM system
Medtronic received FDA approval for its MiniMed Paradigm REAL-Time system, which it described as a milestone toward the development of an artificial pancreas.
The following year, the company expanded the indication for its CGMs to include patients aged 7–17.
2007: Dexcom introduces the Seven CGM
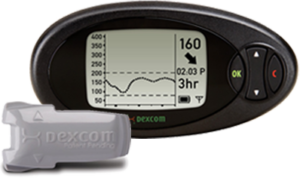
The device was the first approved for up to seven days of use. A model with updated software known as the Seven Plus (shown here) introduced blood sugar trending arrows. The trend arrows can help people with diabetes anticipate in advance when blood sugars are starting to trend high or low.
2007: Medtronic launches the Guardian REAL-Time system with MiniLink transmitter
The standalone personal CGM features a smaller transmitter than preceding models. The transmitter is waterproof to a depth of 8 feet for up to 30 minutes when connected to the glucose sensor.
2008: Abbott launches FreeStyle Navigator CGM in the U.S.
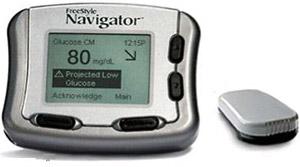
With a sensor life of up to five days, the Navigator provided patients with blood glucose values updated once every 60 seconds.
2008: Medtronic introduces its next-generation professional CGM
Known as iPro, the physician-targeted device was smaller and lighter than preceding models.
2012: Dexcom debuts G4 Platinum
The G4 Platinum offered improved accuracy when measuring hypoglycemic blood glucose values. The device also could work for up to seven consecutive days. The device also featured a consumer-technology-influenced design.
The company would expand its functionality in 2015 with the introduction of the Dexcom Share receiver that transmits G4 Platinum blood glucose data via Bluetooth to a smartphone or iPod Touch app. The functionality allows a patient to share data with up to five designated recipients. Dexcom won permission to use the device in pediatric patients in 2015.
2013: Medtronic introduces second-generation integrated insulin pump/CGM
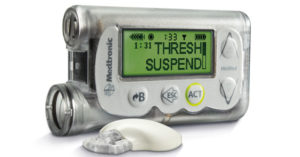
Medtronic’s 530G Enlite was the first integrated insulin pump/CGM to offer a “threshold suspend” feature to manage hypoglycemia. When blood sugar values fall to a predefined low level, the pump stops pumping insulin for two hours.
2015: Dexcom launches G5 Mobile

The G5 Mobile was the first device that enables people with diabetes to manage glucose levels via a smartphone. The device was approved for adults and children as young as two years old. The device also supported the “Share” functionality of its predecessor.
2016: Abbott launches FreeStyle Libre Pro
A professional device, the FreeStyle Libre Pro has a lifespan of up to 14 days. As with other professional devices, glucose data are blinded to the patient. A physician using a FreeStyle Libre Pro reader can access multiple patients’ data. The sensor is water-resistant up to 3 feet for up to 30 minutes.
2017: Medtronic wins approval for closed-loop device
When the FDA approved the Guardian Sensor 3 in 2017, it was the first device to automate insulin delivery via a hybrid closed loop system with the Medtronic MiniMed 670G insulin pump. Other CGM/insulin pumps would follow, such as the t:slim X2 insulin pump integration with the Dexcom G6, which won FDA clearance in 2019.
2017: Abbott launches FreeStyle Libre for U.S. patients
 The device was the first to be factory-calibrated, meaning that it would not require fingerstick testing. The manufacturer referred to the device as a flash glucose monitor rather than a continuous glucose monitor as it required patients to scan the device before seeing data. The device also did not sound alarms for out-of-range glucose readings.
The device was the first to be factory-calibrated, meaning that it would not require fingerstick testing. The manufacturer referred to the device as a flash glucose monitor rather than a continuous glucose monitor as it required patients to scan the device before seeing data. The device also did not sound alarms for out-of-range glucose readings.
The patient-focused device can be worn for 10 days.
2018: Dexcom launches the G6
The FDA approved the Dexcom G6, which featured several upgrades over preceding models. First, its sensor has a 10-day lifespan like the Abbott FreeStyle Libre. It also does not require fingerstick calibration, although such calibration is supported. The device also features more robust interoperability than preceding models, making it compatible with insulin pumps and mobile apps.
2018: FDA approves the Eversense CGM system
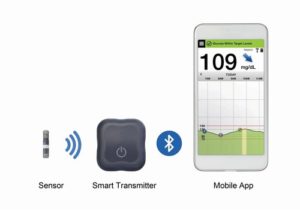
The Eversense CGM from Senseonics provides glucose data every five minutes for up to 90 days via an implantable sensor. A transmitter and mobile app round out the system.
The company’s Eversense XL system, which has a 180-day lifespan, is awaiting FDA approval.
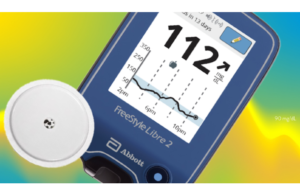
2020: FreeStyle Libre 2 wins FDA clearance
Abbott’s FreeStyle Libre 2, which was introduced in the U.S. in 2020, is an integrated continuous glucose monitoring rather than a flash glucose monitor. It notifies users when glucose levels are high or low without requiring them to scan the device.
The device is the first patient-facing CGM to have a 14-day lifespan.