There’s excitement at Medtronic after the Hugo robotic surgery platform hit a big regulatory milestone.
In 2012, an incubator at Covidien called “Project Einstein” was formed with a focus on combining robotic technology, data and analytics to see what could be created.
About nine years later, the product that emerged from Project Einstein made a major step forward when the Hugo robotic-assisted surgical system received CE mark approval in Europe.
Plenty happened in between then and now, with Medtronic (NYSE:MDT) buying Covidien in one of medtech’s largest acquisitions and Project Einstein’s work carrying on through as Hugo hit its biggest milestone in the regulatory process so far.
Megan Rosengarten, who was part of Project Einstein and now serves as President of Medtronic’s surgical robotics business, said that the excitement brought on by European approval harkens back to the early days of Hugo’s development.
“It goes back to that origin story of how we started this with Project Einstein,” Rosengarten told MassDevice. “It’s the idea and belief that robotic technology could bring quality minimally invasive care to more patients.”
Despite Europe being a highly developed continent, Rosengarten said only approximately 2% of surgical procedures are performed robotically there as adoption of robot-assisted surgery remains in its early stages.
Medtronic already has hospitals, surgeons and customers partnering up with the company, helping Rosengarten and the team to better understand the barriers to adoption in Europe and subsequently create solutions to overcome them.
Rosengarten said there are “a lot of those important partnerships” lined up as there is a real hunger to be the first to use the system in Europe.
“We look for places where there is an appetite and an ability to access technology with a pent-up need to improve patient care by driving more procedures to a minimally invasive approach,” Rosengarten said. “That’s the sweet spot for our belief, that we think we can benefit patients by bringing more minimally invasive therapies to them.
“That’s one of the reasons that it’s like, wow, this is a big deal to get access to this market and impact patients with Hugo.”
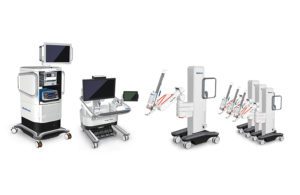
Hugo itself is comprised of a number of components, starting with the surgeon console, from where the robot is driven. The system then has independent robotic arms individually mounted on carts, mainly used for holding the surgical instruments inserted into the patient body.
The wristed instruments are designed to mimic the movement of the surgeon’s hands at the console, holding the same degree of freedom that an actual human hand inside the body would as they perform surgical tasks while guided by the surgeon.
Hugo’s final component is the surgical tower, which Rosengarten likened to the brain or nervous system of the robot. It includes the visualization system and a number of computing systems to analyze and provide data.
Within the tower is Medtronic’s Touch Surgery Enterprise technology, which is applicable for Hugo and for any laparoscopic or robot-assisted surgery from another vendor. A video management solution in its simplest form, Touch Surgery Enterprise automatically records surgical video and delivers it to the cloud, offering a way for surgeons to review certain steps while also being useful for training purposes.
Current indications for Hugo include procedures in urology and gynecology, while the company will be looking at expanding those to at least general surgery, thoracic surgery and bariatric surgery.
“This system was designed for very broad use,” Rosengarten said.
Now that Hugo holds European approval, the next steps include entering what Rosengarten said is the most penetrated market for robotics — the U.S.
The company received FDA investigational device exemption (IDE) approval for Hugo earlier this year and can start its U.S. clinical trial with its first focus set on urological procedures.
While nobody can predict how long the regulatory process in the U.S. will take, Rosengarten said everyone can expect to continue seeing data for Hugo as Medtronic collects it from around the world through its global registry.
Through the collection of patient data from around the world, Rosengarten said Medtronic can learn more about Hugo’s performance while compiling data that will be used for subsequent regulatory submissions beyond urology and gynecology.
“We’ll continue with other indications between the neck, chest, abdomen and pelvis,” Rosengarten said. “You’ll hear more news in the U.S. and elsewhere as that progresses.”