
[weyo/Adobe Stock]
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) is backing a significant shift in the current COVID-19 vaccine strategy: a move from multivalent to monovalent vaccines focusing on the XBB lineage strains. The advisory committee has set its sights on protecting against XBB.1.5 and has jettisoned is support for vaccines with a Wuhan component, which was included as a component of the bivalent vaccine boosters that debuted in fall 2022. “Continuing to include the original Wuhan strain was unnecessary since early strains are no longer in circulation,” said Dr. Amanda Cohn, chief medical officer at the National Center for Immunizations and Respiratory Diseases.
While supporting monovalent vaccines, VRBPAC members acknowledged that the predominant SARS-CoV-2 sublineages could continue to evolve. “We may well find that XBB.1.16 or XBB.2.3 become more dominant than XBB.1.5 in the next month,” said Dr. Stanley Perlman, a voting member of VRBPAC and a professor of microbiology and immunology at the University of Iowa. Perlman, however, noted that this fact likely won’t affect vaccine efficacy, explaining, “this doesn’t matter, though, because we have good data that there’s really good cross reactivity within the S protein.” Even if other XBB sublineages overtake XBB.1.5 in circulation, the high degree of cross-neutralization between the XBB strains will mean the updated vaccines should still provide protection.
VRBPAC projects that updated vaccines will be on the market by September 2023, pending manufacturing timelines and necessary FDA and CDC guidance.
Let’s explore how the monovalent XBB vaccine candidates from Moderna, Pfizer and Novavax have fared in early tests.
Moderna’s XBB.1.5 monovalent vaccine is a contender in COVID vaccine options for fall 2023
In the VRBPAC meeting, Moderna presented substantial data at the VRBPAC meetings, revealing that its authorized bivalent BA.4/5 booster has provided protection against hospitalization and death from COVID-19 during the period when the BA.5 variant was predominant. BA.4 and BA.5 were subvariants of the omicron variant of the SARS-CoV-2 virus in circulation in various parts of the world in 2022. Conversely, XBB.1.5 is a subvariant of the XBB lineage of the virus.
Preliminary data suggest that Moderna’s vaccine containing the XBB monovalent could elicit higher neutralizing antibody responses against XBB subvariants compared to the current bivalent vaccine. “Preclinical data suggests that an XBB containing vaccine is more immunogenic against currently circulating XBB variants than the authorized BA.4/5 bivalent vaccine,” noted Dr. Darin Edwards executive director, COVID vaccines program leader at Moderna in the VRBPAC meeting.
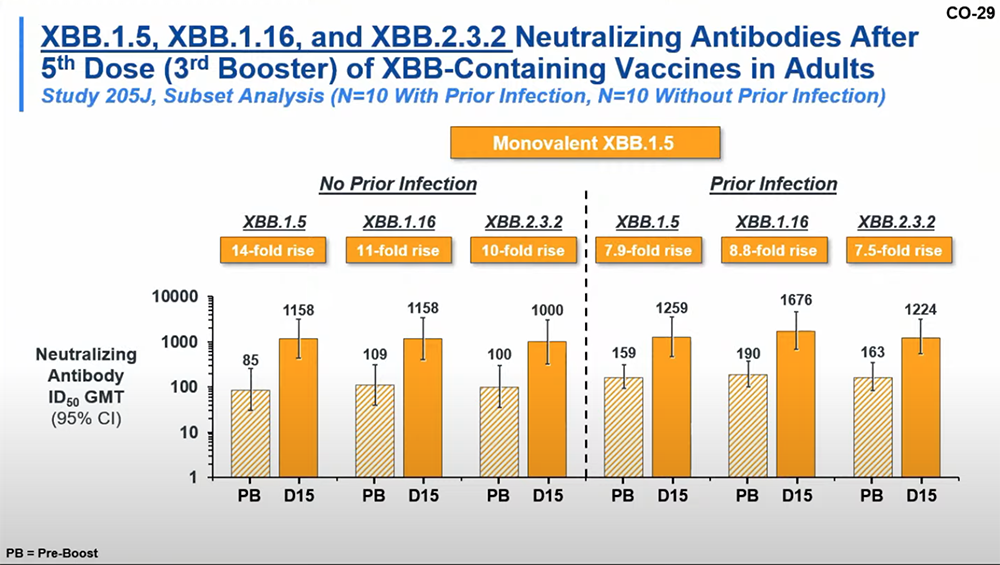
In a trial with 101 participants, researchers found that an XBB.1.5 monovalent vaccine elicited robust neutralizing antibody responses against the XBB.1.5, XBB.1.16 and XBB.2.3 strains. The researchers calculated the geometric mean titers (GMT), an average measure of the antibodies against the SARS-CoV-2 virus in the blood of vaccinated individuals. “Both investigational XBB.1.5 containing boosters increased neutralization against XBB subvariant viruses, with 11- to 33-fold increased neutral neutralizing antibody titers measured after the boost,” Edwards said. “As seen in the previous booster study with XBB.1.5 containing vaccines, only minor differences in neutralization were measured between XBB.1.5, XBB.1.16, and XBB.2.32 viruses.”
Pfizer’s XBB.1.5 monovalent vaccine, another competitor among Fall 2023 COVID vaccine options
In the VRBPAC meeting, Pfizer shared preliminary preclinical data demonstrating that its XBB.1.5 monovalent vaccine elicited the highest neutralizing antibody titers against noteworthy key XBB subvariants, outperforming the current bivalent vaccine. “In the context of two original vaccine doses followed by the current bivalent BA.4/5, we do also see a trend for higher neutralizing titers with the monovalent formulation compared to bivalent,” stated Dr. Kena Swanson, vice president, viral vaccines, vaccine research and development at Pfizer Inc. The company’s clinical data also showed that their bivalent omicron BA.1 vaccine offered improved protection compared to the original monovalent vaccine when the omicron variant was circulating.
In its presentation, Pfizer shared data showing a bivalent omicron vaccine had an effectiveness of 60–75% against COVID-19 hospitalizations in adults under and over age 65, compared to only 10-30% effectiveness for the original monovalent vaccine.

Novavax’s unique position among Fall 2023 COVID vaccine options with its monovalent XBB vaccine
In the recent VRBPAC meeting, Novavax shared clinical data showing significantly reduced neutralization against XBB subvariants with the currently authorized vaccines. The data demonstrated “substantially reduced neutralization against XBB subvariants with currently authorized vaccines, justifying update,” said Dr. Filip Dubovsky, president research and development at Novavax. In particular, Novavax showed geometric mean neutralizing antibody titers against XBB.1 were reduced 45- to 135-fold compared to the ancestral strain depending on vaccine regimen.
Its preclinical data in animal models demonstrated that monoclonal XBB.1.5 or XBB.1.16 vaccines could induce cross-neutralizing antibody responses against XBB subvariants. Novavax reported that in mice, “vaccinating with either XBB.1.5 or 1.16 induces cross-neutralizing responses” against both sublineages.
When used as a booster in primed animals, XBB.1.5 induced comparable antibody responses against XBB.1.5, XBB.1.16 and XBB.2.3. Novavax showed in boosting studies with primed mice and non-human primates that “XBB.1.5 boosts well and induces comparable neutralizing responses to other XBB subvariants,” Dubovsky said.
Novavax’s data: The role of XBB-specific vaccines in neutralizing subvariants
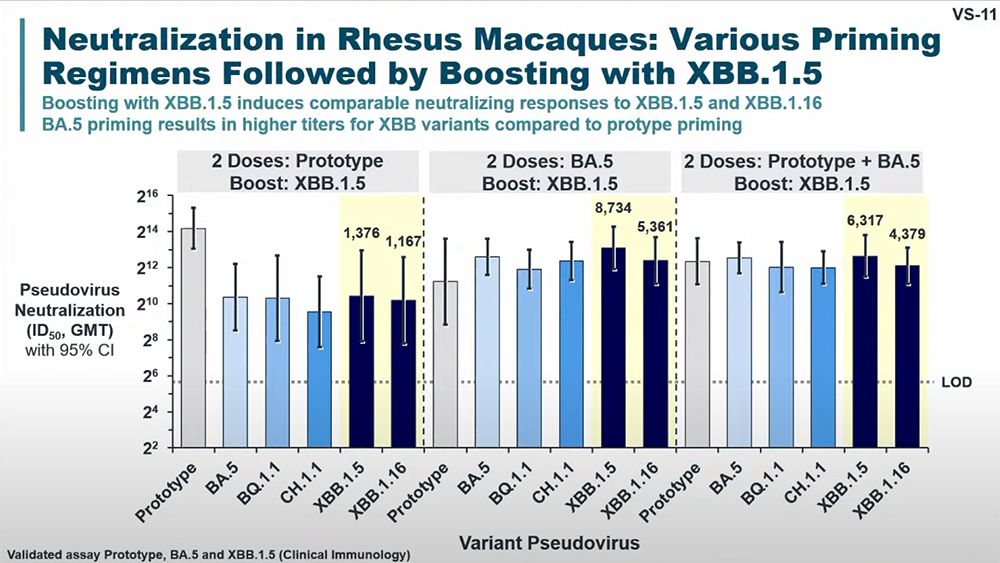
In his presentation, Dubovsky shared results from a non-human primate study testing a variety vaccine prime-boost regimens against the XBB subvariants. He stated that neutralization against XBB subvariants was “substantially reduced with prototype vaccine prime-boost regimen.” Data showed that boosting with either XBB.1.5 or XBB.1.16 “induced high neutralizing antibody titers against XBB.1.5, XBB.1.16, and XBB.2.3.2, ranging from geometric mean titers of 338 to 741.”
Specifically, Novavax found that an unadjuvanted XBB.1.5 boost achieved robust geometric mean neutralizing antibody titers against XBB.1.5 (GMT 538), XBB.1.16 (GMT 338) and XBB.2.3.2 (GMT 476).
Novavax, one of the first companies to be involved in Operation Warpspeed, has struggled to capitalize from the pandemic. Its stock peaked at $290.18 on February 5, 2021, but has since fallen to under $10 per share. The company has laid off about one-quarter of its workforce. In March 2023, the company issued a “going concern” warning to investors referencing its operational struggles.