 Founded in 2011, Caribou Biosciences is a pioneer in the development of CRISPR genome editing technologies, a field honored with the Nobel Prize in Chemistry in 2020. Co-founded by Jennifer Doudna, Ph.D., one of the Nobel laureates, and CEO Rachel Haurwitz, and two other CRISPR pioneers, the company has raised over $800 million in funding, including significant investments from industry giants like Pfizer. In 2021, the company entered the clinic with their lead program and completed a successful $350 million IPO.
Founded in 2011, Caribou Biosciences is a pioneer in the development of CRISPR genome editing technologies, a field honored with the Nobel Prize in Chemistry in 2020. Co-founded by Jennifer Doudna, Ph.D., one of the Nobel laureates, and CEO Rachel Haurwitz, and two other CRISPR pioneers, the company has raised over $800 million in funding, including significant investments from industry giants like Pfizer. In 2021, the company entered the clinic with their lead program and completed a successful $350 million IPO.
“Today, we’re laser focused on using Caribou’s next generation CRISPR technology, which we call chRDNA technology to advance a pipeline of wholly owned off the shelf CAR-T and CAR-NK cell therapies,” said Haurwitz in a recent interview at the JP Morgan Health Care conference.
CRISPR: From lab to clinic in about a decade

Rachel Haurwitz, Ph.D., CEO of Caribou Biosciences
Rachel Haurwitz, Ph.D., CEO of Caribou Biosciences CRISPR itself remains a relatively new technology with the FDA approving the first CRISPR-Cas9-based gene therapy, Casgevy (exagamglogene autotemcel or exa-cel), in December 2023. “The technology is about a dozen years old,” Haurwitz said. The approval of Vertex Pharmaceuticals’ and CRISPR Therapeutics’ Casgevy also marked one of the first gene therapies to win FDA approval for sickle cell disease. More recently, Casgevy won FDA approval for transfusion-dependent beta thalassemia (TDT).
The breakthroughs are noteworthy considering that the seminal CRISPR-Cas9 research paper by Jennifer Doudna and collaborators was only published in Science in 2012. “I’m not aware of any other major technology platform that was able to go from cool science to an approved product that quickly,” Haurwitz said.
Caribou aims to forge new paths in hematologic cancer care with CAR-Ts
To date, Caribou Biosciences has three clinical-stage allogeneic CAR-T cell therapies in phase 1 clinical trials, each targeting different hematologic malignancies. The company’s lead program, CB-010 for non-Hodgkin lymphoma, has been in a clinical trial since 2021. “In mid-2023, we were able to share some very encouraging dose escalation data from 16 patients as part of our ANTLER phase 1 trial for CB-010,” Haurwitz noted. The results showed durable responses comparable to those seen with autologous CAR-T therapies. This positive outcome led the company to ask the FDA to focus on second-line patients earlier than typical, which the agency agreed with.
In the ANTLER phase 1 trial, in the dose escalation portion in 16 patients, a 94% overall response rate and 69% complete response rate were observed, with 44% of patients achieving a complete response rate at six months or later.. In addition, CB-010 has received Regenerative Medicine Advanced Therapy (RMAT), Fast Track, and Orphan Drug designations from the FDA.
CAR-Ts and blood cancers outcomes
Since their debut in 2017, autologous CAR-T cell therapies have significantly altered the treatment outcomes for patients with non-Hodgkin lymphoma (NHL). “I would argue that for the kinds of patients we’re trying to serve with non-Hodgkin lymphoma, the advent of autologous CAR-T therapies, several years ago, dramatically changed outcomes for that patient population,” Haurwitz said. The therapies involve genetically modifying a patient’s T cells to recognize and attack lymphoma cells. The most common targets for these therapies are the CD19 and CD22 proteins found on the surface of B-cell lymphomas.
Haurwitz mentioned that in conversations with key opinion leaders, the topic of treatment options available for cancer patients in their clinics and how they prioritize those treatments over time. “They just kept reminding us that CAR-Ts are the only approach with potentially curative intent,” she said. “You’re probably not going to cure cancer with an antibody-based therapy, but we could maybe with CAR-T,” Haurwitz said. “For that reason, the platform has significantly changed non-Hodgkin lymphoma treatment. The field has also seen some incredible outcomes for patients with autologous CAR-Ts in multiple myeloma.”
Pursuing allogeneic CAR-Ts
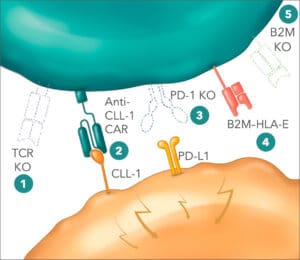
The allogeneic CAR-T cell therapy candidate CB-012 targets CLL-1-positive acute myeloid leukemia cells.
Yet autologous treatments come with challenges in scaling up manufacturing and meeting demand. This has motivated Caribou’s pursuit of allogeneic CAR-T platforms. Caribou’s pipeline of allogeneic CAR-Ts include CB-011, an allogeneic anti-BCMA CAR-T cell therapy for treating patients with relapsed or refractory multiple myeloma (r/r MM), which employs Cas12a chRDNA genome-editing technology in manufacturing and involves multiple edits including a B2M–HLA-E-peptide fusion insertion. CB-011 is the focus of the company’s CaMMouflage Phase 1 clinical trial. Next up is CB-012, which targets relapsed or refractory acute myeloid leukemia (r/r AML), CB-012 is an allogeneic anti-CLL-1 CAR-T cell therapy, also using Cas12a chRDNA technology and including a PD-1 knockout and B2M–HLA-E insertion. CB-012 is being evaluated in the AMpLify Phase 1 trial.
“Both Cas9 and Cas12a chRDNAs are very good at gene knockouts,” Haurwitz said. “Cas12a is the belle of the ball for doing gene insertions. And so we’re able to achieve in the case of CB-011 four edits, and in the case of CB-012, five edits with very high efficiency while maintaining genomic integrity. So it’s a really nice balancing act between getting the edits you want and avoiding the ones that you don’t want.”
Caribou Biosciences also focusing on novel CAR-NK therapy for solid tumors
Finally, Caribou’s CB-020 is currently in preclinical development. Based on Caribou’s CAR-NK platform, CB-020 represents a novel genome-edited product candidate derived from its proprietary induced pluripotent stem cell (iPSC) natural killer (iNK) cell therapy platform. “This is our strategy for solid tumors, where we recognize that the complexity of the biology of getting a cell therapy to the tumor and into the tumor, maintaining sufficient activity once it’s there, will require quite a lot of genome editing and armoring of the cell therapy,” Haurwitz said.
Engineered using Caribou’s Cas12a chRDNA genome-editing technology, CB-020 expresses a ROR1-specific chimeric antigen receptor (CAR). ROR1, a receptor tyrosine-kinase-like antigen prevalent in various solid tumors, promotes tumor growth, survival and metastasis. The ROR1-specific CAR is intended to boost NK cell antitumor activity through increased specificity and function. Preclinical studies have already demonstrated that iPSC-derived anti-ROR1 CAR-NK cells significantly reduce tumor burden compared to control iPSC-NK cells without the CAR.
Founded as a diverse company
Haurwitz noted that Caribou has made efforts to foster gender diversity and equity within the company. While scientific advisory boards are typically male-dominated, Caribou’s Scientific Advisory Board is nearly half female. “And our leadership team is a modest majority of women,” Haurwitz noted. Across the entire company, the gender ratio is almost equal, slightly skewing towards 53% women. “Inside our four walls, I think things look very different from outside our four walls, especially from a gender perspective in the industry,” Haurwitz said. “We’re trying to include as many diverse voices at the table as we can.”
Haurwitz emphasized that Caribou has not used quotas to achieve this level of gender balance. “We’ve never opened a role and said, ‘This has to be filled by a woman,’ or ‘This has to be filled by a person with this kind of background,’” Haurwitz said. “We’re always looking for the absolute best candidates we can find.”
Haurwitz noted that, in the beginning, the company had a 50/50 split of men and women. In addition to herself and Jennifer Doudna, the other cofounders include Martin Jinek, a former postdoctoral fellow in Doudna’s lab, now at the University of Zurich, and James Berger, who is now a professor in the department of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine. The company’s early even gender split “just helped us propagate a more diverse organization from the get-go,” Haurwitz said.