(Image by Sam Moqadam on Unsplash)
Reports of severe allergic reactions to COVID-19 vaccines have made consistent headlines in recent weeks. Still, such anaphylactic reactions are rare, occurring in approximately 11 out of every million doses for the vaccine from Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX), according to CDC.
While that is roughly 10 times the flu vaccine rate, COVID-19 vaccines so far have been generally well-tolerated in the vast majority of patients.
“The anaphylaxis rate for COVID-19 vaccines may seem high compared to flu vaccines, but I want to reassure you that this is still a rare outcome,” said Dr. Nancy Messonnier, the director of the CDC’s National Center for Immunization and Respiratory Diseases in a recent call with reporters.
More recent CDC data suggests a lower rate of anaphylactic reactions of 5.5 per 1 million injected vaccine doses.
Anaphylaxis, a severe and potentially life-threatening allergic reaction, is generally responsible for approximately 200,000 emergency-room visits annually.
Patients with severe allergies were screened out of Phase 3 COVID-19 vaccine trials from Pfizer-BioNTech and Moderna (NSDQ:MRNA). Those trials cited hypersensitivity reactions rather than anaphylaxis.
Because most reactions occur soon after vaccination, CDC has recommended that vaccine sites have an epinephrine injection device available.
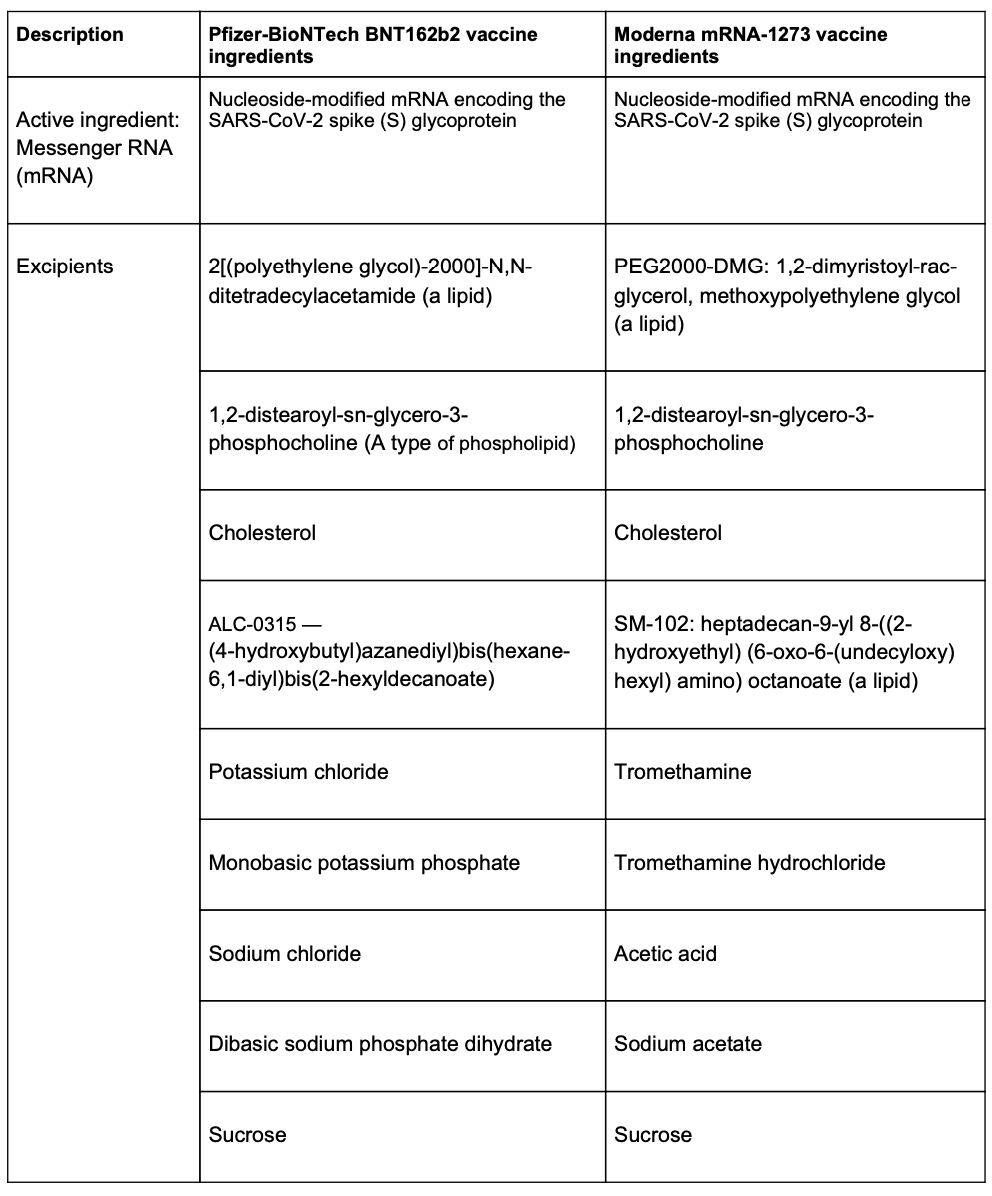
The reason for the reactions is still a mystery. Neither the Pfizer nor the Moderna vaccine contains common allergens such as eggs, latex, gelatin or preservatives.
One recent paper posits that polyethylene glycol plays a role in such allergies. Polyethylene glycol is used in some foods, cosmetics and medicines. Until recently, it had not been used in commonly used vaccines.
Some pundits have speculated that nanoparticles may be responsible for allergic reactions. Nanoparticle vaccines first appeared in 1986 with a virus-like particle vaccine for hepatitis B.
Anaphylactic reactions have been reported for both the Pfizer-BioNTech and Moderna vaccines, and forms of nanoparticles and polyethylene glycol are present in both vaccines.
Although anaphylactic reactions to polyethylene glycol have not been widely reported, in recent years, there has been a rise in complaints about allergic reactions related to the chemical from drugs and personal hygiene products.
Here are the ingredients in the two COVID-19 currently available in the U.S.: