PROTACs, or PROteolysis TArgeting Chimera, emerged as novel therapeutic modalities in 2001 but have only begun emerging from clinical trials (PROTAC® is a registered trademark of Arvinas. In this article, PROTAC specifically refers to the abbreviation of PROteolysis TArgeting Chimera as therapeutic modalities). PROTACs’ unique structures and modes of action (MOA) differentiate them from conventional small molecules and present exciting and unprecedented therapeutic opportunities. But questions remain about PROTACs’ broad-scale utility, given some of the pharmacological challenges scientists have uncovered during preclinical and clinical research.
In the first of a two-part series, we will showcase PROTACs’ unique structure and function, and highlight the challenges and strategies that PROTAC drug sponsors and developers should anticipate from the perspective of DMPK (drug metabolism and pharmacokinetics) studies. The second article in this series will dig deeper into the specific solubility and permeability challenges PROTAC drug developers face.
PROTAC structure and function
PROTACs are bifunctional molecules that leverage the body’s internal protein disposal system (i.e., ubiquitin-proteasome system) to target and degrade disease-causing proteins. Like its mythological three-headed namesake, a PROTAC consists of three structural parts (Figure 1): a ligand that binds to the ubiquitin-protein ligase E3 (i.e., E3 ligase recognition moiety); a ligand that binds to a target protein meant for degradation; and a covalent linker connecting the two ligands. PROTAC induces E3 ligase to label the target protein with ubiquitin by bringing the E3 ligase and the target protein into proximity, promoting the degradation of the target or disease-causing protein PROTAC molecules fall off after the target protein degradation.
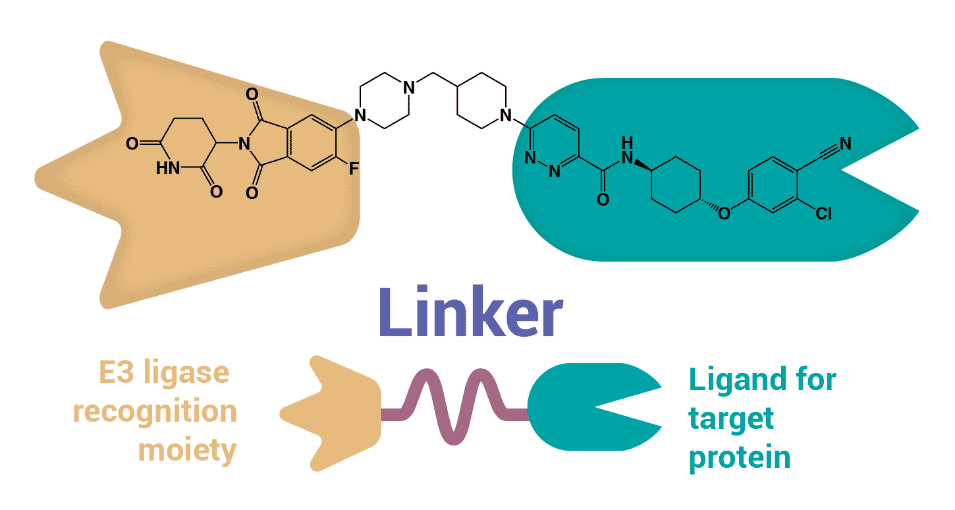
Figure 1: Schematic of typical PROTAC structure and an example (ARV-110).
This ubiquitination process allows PROTACs to access previously inaccessible proteins and treat diseases once thought to be untreatable. In addition, their ability to selectively degrade pathological proteins provides a significant advantage over other small molecules and has led to a rapid rise in popularity in drug development.
Unlike occupancy-driven pharmacology, which introduces high concentrations of an inhibitor to block problematic protein function, PROTACs use an event-driven MOA that degrades protein at the cellular level. This process delivers sustained destruction of disease-causing proteins that profoundly impact therapies for cancer, immune disorders, viral infections and neurodegenerative diseases.
Protein degradation in PROTAC drugs is also an iterative process rather than a competitive one. PROTACs are delivered in very low doses, making them less susceptible to inducing protein overexpression or mutations of the target protein. This low dosage level reduces the likelihood of drug resistance to PROTACs in targeted tumor therapies.
The DMPK challenges inherent in PROTAC drug development
Despite PROTACs’ promising future, developing these molecules still comes with multiple challenges. The second article in this series will dig deeper into permeability and solubility challenges, but these DMPK challenges represent significant hurdles to overcome when developing PROTAC drugs.
- There is no specific guidance from regulatory agencies (U.S. FDA or ICH) about addressing the specific DMPK challenges. In practice, the guidance for small molecule drugs is generally referred to on a case-by-case basis, but PROTACs are significantly different from traditional small molecules.
- PROTACs’ unique structure makes it difficult to meet Lipinski’s Rule of Five designed for small molecules. These drugs’ molecular weight usually falls within the 700-1000 Da range with poor solubility. They also struggle to meet Lipinski’s other rules for lipophilicity, hydrogen bond donors or acceptors and polar surface area.
- PROTAC drugs have poor permeability, resulting in poor druggability for oral administration, and the correlation between in vitro and in vivo permeability is weak. Also, in vitro permeability evaluation systems for small molecules are usually not suitable for PROTACs.
- PROTAC drugs have shown a “hook effect” (i.e., at high concentrations, a target protein-PROTAC or E3 ligase-PROTAC binary complex is formed competitively, leading to reduced efficacy or toxic reactions). This requires the drug research community to understand more clearly PK/PD characteristics for PROTAC drugs and the proper dose ranges.
- PROTACs’ metabolites, especially the linker-cleavage metabolites, may compete for binding to targets or E3 ligases. Similar to the “hook effect,” such binding reduces efficacy. Therefore, metabolite profiling and identification is a crucial part of its preclinical screening, which is important not only for selecting appropriate toxicology species but also for understanding the dose-response relationship.
Strategy for conducting a DMPK study for PROTACs
The clear challenges associated with PROTACs mean designing a preclinical evaluation strategy requires consistent optimization of physiochemical and DMPK properties (Figure 2).
- Early Screening Stage: Need to be performed by first characterizing the in vitro properties of PROTAC molecules, which encompasses physicochemical properties, permeability, metabolic stability, protein binding, and CYP inhibition.
- Optimization Stage: The focus is on improving the metabolic clearance and solubility of PROTAC, combined with the PK properties of extravascular drug delivery, to better understand the DMPK properties of PROTAC, such as absorption and metabolism. PROTACs’ permeability, although important for absorption, is unlikely to be significantly improved due to its structure and high molecular weight. Orally administered PROTACs will require more attention to solubility and metabolic stability during the optimization phase.
- PCC Stage: PROTAC molecules with potent and better oral bioavailability can be used in further PK/PD studies to obtain a more in-depth exposure-response relationship.
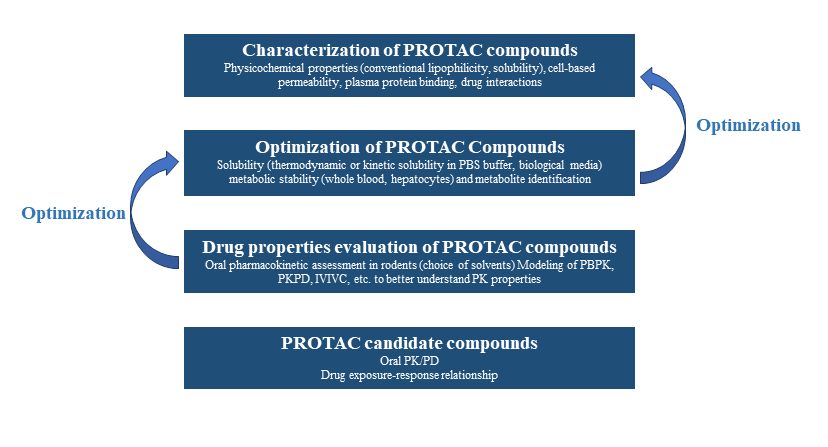
Figure 2. Strategy and process of conducting DMPK study for PROTAC drugs
Cause for optimism
Drug sponsors and developers continue with discovery efforts and find success despite developmental challenges. The benefits of bringing PROTACs to market outweigh the time and effort needed to get there. And there is cause for optimism. For example, U.S.-based Arvinas has three candidate models showing great potential. ARV-110 (prostate cancer) and ARV-471 (breast cancer) are in clinical phase II, and ARV-766 (prostate cancer) is in clinical Phase I. Moreover, Kymera also has three promising candidates in clinical Phase I, i.e., KT-474 for Multiple Immuno-inflammatory Diseases and KT-333 and KT-413 for cancer treatment. It is fair to say all eyes are on these clinical trials.
As PROTAC investigations evolve, scientists will undoubtedly understand how to mitigate some of the challenges encountered today. In addition, there are some strategies developers and sponsors can use to help in these efforts.
Screen compounds with caution. Traditional PK screening strategies may not be suitable for PROTACs today. Therefore, sponsors, developers and scientists must exercise caution in screening compounds using only in vitro data.
Choose E3 ligases carefully. An E3 ligase’s ubiquitination is based on the target protein to which it binds. This tissue fidelity creates an inherently challenging hurdle in matching a specific target protein with its corresponding E3 ligase. Scientists have discovered more than 600 E3 ligases in the human genome, so there are myriad combinations. Thus far, much of the research around PROTACs focuses on the E3 ligases CRBN, VHL, IAPs and MDM2. Likewise, target research concentrates mainly on PEG and alkyl. As scientists continue exploring E3 ligase-ligand partnerships, more PROTAC candidates will likely emerge.
Seek out new protein targets. There has been significant research into targeting estrogen receptors (ER) and androgen receptors (AR). The PROTACs that have seen the most success when targeting these proteins include Arvinas’ three drugs in clinical trials. There are very few instances of degraders reported for undruggable targets. To truly target undruggable diseases, scientists must continue exploring undruggable proteins.
A final word
The excitement around PROTAC drugs is understandable. Their unique mechanism of action and rapid rate of progression through clinical trials is evidence of their immense potential to target the worst diseases. But PROTAC research has encountered some significant hurdles even in the specific field of DMPK that it must overcome before this vast potential can be unlocked. Open communication and timely collaboration between laboratory testing partners, academic institutions and drug developers/sponsors is the best way to advance the field and deliver these therapeutic options.
Dr. Liping Ma, Ph.D. of Pharmaceutical Analysis, an expert in PROTAC pharmacokinetic evaluation, is now a senior study director in the DMPK Service Department of WuXi AppTec. She has over 10 years of research experience in preclinical and clinical pharmacokinetics and extensive experience in the preclinical development of drugs. She successfully supported more than 20 new drug IND applications globally.