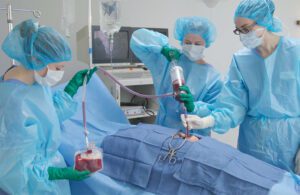
Sisu Global Health’s autotransfusion device, Hemafuse, can collect blood lost by a patient for reuse during surgeries. [Photo courtesy of Sisu Global Health]
The developer of an autotransfusion device for collecting and cleaning a patient’s lost blood is sending hundreds of units to Ukraine as casualties climb from the Russian invasion.
Baltimore-based Sisu Global Health has already sent 1,005 of its Hemafuse devices to hospitals in Kyiv and is planning a third shipment of 500 more any day now, co-founder and President Gillian Henker said.
“We were talking amongst our management team and our shareholders, and they were saying we really want to do something for Ukraine,” Henker said in an interview with Medical Design & Outsourcing. “And we really see Hemafuse as a great fit.”
After those first conversations in March, the company raised $110,000 through nonprofit FINCA International and other partners to send the kits to Ukraine. They hope to be able to send another 500 in a fourth shipment, Henker said.
Hemafuse is a handheld device that uses a Yankauer suction tip to salvage blood from a patient with internal bleeding so the blood can be re-used in the patient if donor blood is unavailable or in limited supply. The device can be reused about 25 times.
The device can be used for ruptured ectopic pregnancies, burst spleens and livers, coronary artery repairs, total hip replacements, abdominal aneurysms and myomectomies. A patient who receives their own blood won’t reject the blood or be infected by someone else’s bloodborne pathogen.
“Your own blood is better than someone else’s,” Henker said. “With this device, you want to make sure that it maintains the quality of that blood and gives that best blood back to that same patient.”
Blood is in high demand on and off the battlefield. The American Red Cross declared its first-ever blood crisis this year, citing a decline in donations in the COVID-19 pandemic.
“There are community hospitals in the U.S. that are in rural areas that don’t have blood banks in their facilities,” Henker said. “They might only have one or two units on hand for emergencies, especially certain components that help with clotting or stopping the bleeding. We see Hemafuse as a great product to have on hand for more rural community hospitals, and what you might think of as rural or community could just be the next county or two counties over from a major city.”
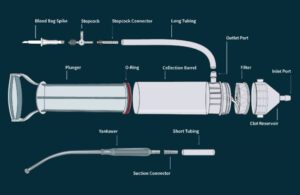
The Hemafuse autotransfusion device is made of high-heat plastics. [Photo courtesy of Sisu Global Health]
How the Hemafuse autotransfusion device was developed and designed
Henker said she was in a Sub-Saharan Africa hospital in 2010 when she was asked her blood type for a maternal patient who needed a transfusion. Henker wasn’t a match — someone else was, and the mother’s life was saved — and “that’s where the idea and the invention of Hemafuse came about,” she said.
Four years later, Henker and co-founders Carolyn Yarina and Katherine Kirsch launched Sisu Global Health. They interviewed doctors, nurses and techs in West Africa to learn more about their challenges before developing prototypes. Early prototypes were 3D-printed and super-glued together, with Henker testing fluid dynamics in her bathtub.
“There were a number of different fun things with roommates walking in and me being like, ‘Hey, no worries. I’m making a medical device here,’” Henker said.
Henker and Yarina — engineers who met at the University of Michigan — started with several different designs, but the device’s current form of an oversized syringe won for simplicity.
“The giant syringe was a really big breaking point for the team. You see it and you have an intuition on how it should be used,” Henker said. “Especially with our shipments that we’ve had to Ukraine, we haven’t had the most interaction with those clinicians at the moment. We’ve translated of course our instructions for use and our manuals, but we are relying on the design of the device to help it be simple for them to intuitively use it in the field.”
The Hemafuse pump is mainly injection molded with high-heat plastics in the U.S., with one part made in Asia and assembly in Baltimore by Harbor Designs & Manufacturing.
The filter is a polyester-like dual mesh that removes blood clots, particulate, bone fragments and tissue. The filter is folded in a specific way to increase surface area and flow, Henker said.
“The more surface area you have, the greater flow can go through it and our device can get you several units of blood in a couple of minutes,” she said.
Sisu Global Health launched the device in Kenya and Ghana in 2019 and won FDA 510(k) clearance last summer.

Sisu Global Health co-founder and President Gillian Henker [Photo courtesy of Sisu Global Health]
Design challenges and lessons learned
Henker learned some important design lessons through the development of Hemafuse.
“Do the best you can to think about what you need to do now to get to the next stage, not how do you go all the way to the end,” she said.
She offered the example of Hemafuse’s 3D-printed prototypes. “What do we need to get on board with that stage of development — customer interest, medical needs identified, different proof of concepts modeling, even if it’s not the most sophisticated — to then get to the next stage of funding? With a 3D-printed prototype, we were able to get a grant that launched us into being able to do more manufacturing, biocompatibility and sterilization testing, which allowed us to do our first clinical study.”
The hardest challenge of designing Hemafuse was maintaining simplicity throughout the device and limiting the number of parts, work that continues to this day.
“Probably within the next year, we’re going to be looking at streamlining our production and making things easier for some of our users, especially if they need a little bit more time to put together the device,” Henker said. “We’re looking at increasing that use factor and streamlining production.”