 Rocket Pharmaceuticals (Nasdaq:RCKT) is a late-stage clinical biotech focusing on developing gene therapies for rare pediatric diseases with high unmet need.
Rocket Pharmaceuticals (Nasdaq:RCKT) is a late-stage clinical biotech focusing on developing gene therapies for rare pediatric diseases with high unmet need.
In 2016, that focus drew in Kinnari Patel, president and COO and Jonathan Schwartz, chief medical officer of the company.
“My passion has been making rare disease drug development more efficient and having more therapies available per patient,” Patel said. “That’s always been my goal since I started my student rotation at the Office of Orphan Product Development at FDA in 2004.”
While safety concerns have recently surfaced in a limited number of gene therapy clinical trials, the field’s promise to develop curative therapies for rare diseases remains captivating.
In fact, Schwartz said the opportunity to work on potentially curative therapies was part of his reason for joining Rocket. “It was a chance to work on cutting-edge science addressing the cause of diseases as opposed to some epiphenomenon, which is, unfortunately, the way most of our medications work,” he said.
It also helped that Patel and Schwartz also worked with Dr. Gaurav Shah, Rocket Pharmaceuticals’ CEO, in prior roles.
“In 2015, Gaurav called me, saying, ‘Hey, I’m thinking of starting a company focused on rare diseases in pediatrics — mostly in gene therapy,” Patel recalled.
Patel recalls being excited about the focus on potentially curative therapies for rare disease in pediatrics. “The only advice I had for him was, ‘If you start the company, the end game can’t be an M&A. Let’s do this the right way for the long-term impact of the patients,’” she said. Shah agreed. “And a few months later, I ended up joining the company,” Patel said.
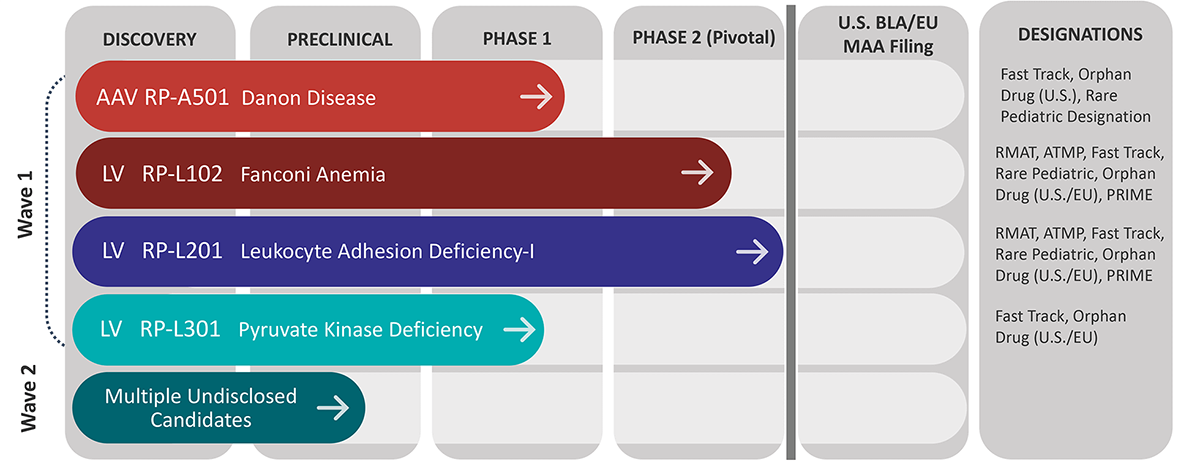
An overview of Rocket’s pipeline
The company currently has lentiviral vector (LVV)-based gene therapy programs for Fanconi anemia (FA), leukocyte adhesion deficiency-I and pyruvate kinase deficiency. Rocket also has a clinical program featuring adeno-associated virus (AAV)-based gene therapy for Danon disease, a pediatric heart failure condition.
“There is a compelling clinical proof of concept in each of these disorders,” Schwartz said. “We are, in some cases, curing or radically transforming these underlying diseases.”
[Image courtesy of Rocket Pharma]
The furthest along is RP-L201, which is in a registration trial for leukocyte adhesion deficiency-I (LAD-I). The disorder is a devastating immunodeficiency resulting from a mutation that codes for a critical receptor on white blood cells. Patients with LAD-I can have recurring bacterial and fungal infections as well as periodontitis. “Children with the severe variant of this disease very infrequently survive early childhood,” Schwartz said.
Next in line is RP-L102 for Fanconi anemia, an inherited bone marrow failure syndrome. The disorder can result in bone marrow failure, a predisposition to cancer and early mortality. About 80% of Fanconi anemia kids will have a severe bone marrow failure in the first decade of life,” Schwartz said. “While an allogeneic bone marrow transplant is curative, it comes with considerable toxicities, and it’s not necessarily available to everyone.”
Rocket’s Fanconi anemia program has been successful at arresting bone marrow failure in a majority of patients. “We can prevent patients’ blood counts from declining and restore their blood cells’ ability to repair DNA,” Schwartz said. “The therapy becomes a very low toxicity intervention that can prevent bone marrow failure and prevent the need for bone marrow transplant.”
In addition, Rocket has a Phase 1 study underway for RP-L301 in pyruvate kinase deficiency (PKD), a red blood cell disorder that causes hemolytic anemia. The severe variant of PKD can be debilitating. Rocket has treated two patients with severe anemia in the adult cohort of its Phase 1 study. “Both patients tolerated the therapy extremely well,” Schwartz said. “And 18 to 24 months after treatment, both have normal hemoglobin levels in the 13 and 14 g/dL range. They’re transfusion independent and essentially living normal lives.”
For context, anemia is defined as having hemoglobin levels of <12.0 g/dL in women and <13.0 g/dL in men.
RP-A501 is the company’s AAV-based program for Danon disease, a disorder of autophagy. People with Danon disease have cells that cannot dispose of intracellular debris, including senescent proteins and mitochondria. As a result, the disease “results in the most aggressive cardiomyopathy that’s ever been described,” Schwartz said. “In male patients, the median age of death is 19 years.” Females live about two decades longer. Because Danon disease is an X-linked disorder, females with Danon disease have at least one functional chromosome with a lysosome-associated membrane protein 2 (LAMP-2) protein.
The Phase 1 study of RP-A501 began with an adult cohort before moving into a pediatric cohort. “We have seen sustained benefit across multiple endpoints, including biomarkers of heart failure like B-type natriuretic peptide (BNP) and troponin, as well as cardiac structural components as evidenced on echocardiogram,” Schwartz said.
Patients with Danon disease have thick hearts that continue to thicken throughout adolescence. “We’ve been able to stabilize and improve that thickening in many of our patients,” Schwartz said. The company has also seen functional benefits such as improvement in New York Heart Association Functional Classification. “We’ve also now shown that the safety profile in the pediatric cohort has been extremely favorable — especially with an enhanced immunomodulatory regimen,” he added. “So the patients in the pediatric cohort and likely in the Phase 2 going forward, will hopefully have very limited safety concerns or side effects.”
Not a platform company
Rocket Pharmaceuticals is an atypical gene therapy company. For one thing, it bills itself as platform-agnostic. “We’re not a platform company,” Patel said.
That is, instead of starting with a platform and looking for diseases to target, the company starts with a study of a disease and its genetic and cellular underpinnings. “For us, the commitment is to address the disease, Patel said. “It’s secondary to us if that’s done through ex vivo lenti or our AAV platform.”
Patel also says the company is unique in the depth of feedback it solicits from scientists, patients, patient advocates and treating physicians. The feedback from all of those parties has helped the company create clinical studies that allow the company to identify the benefits and risks of a given program.
Patel also notes that the company focuses on chemistry, manufacturing and controls (CMC) at the outset. “A lot of times in gene therapy, CMC is an afterthought,” Patel said. “You don’t want to do CMC comparability changes. You want to eliminate as many differences as possible in your product.”