 The biopharmaceutical company IMV (NSDQ:IMV) has recently revealed promising data related to its lead immunotherapy as a potential ovarian cancer treatment. In the Phase 2 DeCidE1 trial, the immunotherapy DPX-Survivac with intermittent low-dose cyclophosphamide showed durable anti-tumor activity in patients with recurrent, advanced ovarian cancer.
The biopharmaceutical company IMV (NSDQ:IMV) has recently revealed promising data related to its lead immunotherapy as a potential ovarian cancer treatment. In the Phase 2 DeCidE1 trial, the immunotherapy DPX-Survivac with intermittent low-dose cyclophosphamide showed durable anti-tumor activity in patients with recurrent, advanced ovarian cancer.
To learn more about DPX-Survivac’s potential, we reached out to IMV Chief Medical Officer Joanne Schindler. In the following interview, Schindler touches on the standard of care for treating recurrent ovarian cancer and provides an overview of the potential of the T-cell immunotherapy for treating a range of cancers.
Could you highlight some of the main challenges in treating recurrent ovarian cancer?
Schindler: In recurrent ovarian cancer, platinum-based chemotherapy is the standard of care option for patients who have responded to prior platinum therapy and for whom recurrence was not rapid (e.g., > 6 months from the end of therapy). However, the usual course of disease is for treatment-free intervals to become progressively shorter after each relapse and for patients to eventually develop resistance to platinum-based therapies. During this time, patients typically experience worsening symptoms and cumulative toxicities with each new treatment line, making maintenance of quality of life an important component of treatment decisions. Both current and potential new targeted therapies such as PARP inhibitors, anti-angiogenesis agents, ADCs, gene therapies and checkpoint inhibitors come with significant side effects that can be challenging to tolerate. New treatment options are needed not only to improve response rates but to delay time to next treatment, alleviate symptoms and maintain quality of life while limiting side effects from treatment.
How can the DPX platform help address such challenges?
Schindler: The DPX platform is a novel formulation that delivers cancer-specific targets directly to antigen-presenting cells, resulting in a T cell response that can be sustained in circulation with repeated injections over an extended period. Data from the DeCidE trial showed that DPX-Survivac, which targets the cancer antigen, survivin and intermittent low-dose cyclophosphamide, led to the generation of survivin-specific T cells that were sustained over time. Long-lasting clinical responses were reported, with some responders showing prolonged duration of clinical benefit for more than a year. The most common treatment-related toxicities were injection-site reactions that were mostly mild to moderate. Systemic reactions were limited in number and mostly low grade as well. We anticipate that future studies will show that this safety profile and the ease of administration of DPX-Survivac will translate into improved quality of life.
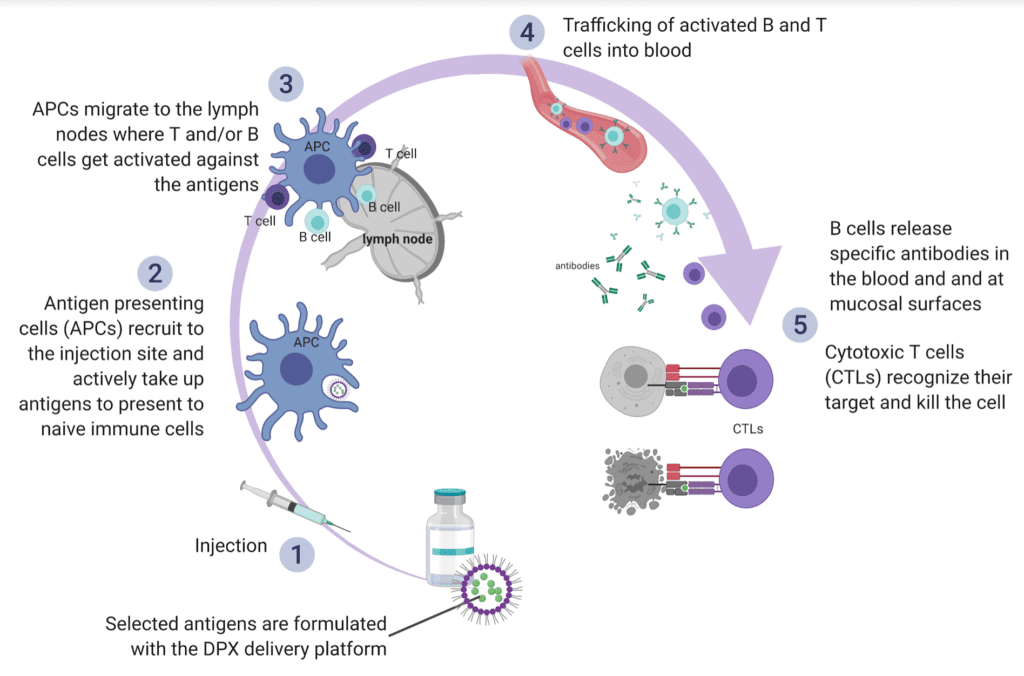
A diagram of the MOA – DPX platform. Image courtesy of IMV
Ovarian cancer is a challenging disease to treat since it is usually detected at late stages. The standard first-line treatment for these patients consists of debulking surgery and a platinum-based chemotherapy regimen. Unfortunately, most patients will face recurrence that invariably ends in disease that is resistant to standard chemotherapies. Life expectancy for patients whose disease is refractory or resistant to platinum-based therapy is typically less than one year.
In the DeCidE trial, patients could have platinum-sensitive or resistant, recurrent ovarian cancer. However, most patients enrolled in the study were resistant or refractory to their last line of platinum-based chemotherapy. The majority of patients had received three or more lines of therapy, including a PARP inhibitor. Additionally, most patients had progressed within six months of their last line of treatment. In this challenging setting, it is promising to report that the median overall survival had not been reached in this cohort since more than 50% of patients remained alive at the time of the presentation (Dec. 3, 2020). We continue to follow the patients as the data matures over time.
IMV has a large amount of biomarker data. Where is the company now in interrogating that data?
Schindler: Late last year, we initiated extensive analyses from our ongoing exploratory translational research program using data from tumor and blood samples obtained from our DeCidE study. This study evaluates DPX-Survivac and intermittent low-dose cyclophosphamide (CPA) in patients with advanced, recurrent ovarian cancer. Statistical analyses are in progress, and we believe it may highlight trends that we will want to further investigate in a larger cohort.
Any recent news regarding discussions with regulators you can share regarding the use of DPX-Survivac for ovarian cancer?
Schindler: We consistently communicate with regulatory authorities in the U.S. and Canada regarding product development activities, including ongoing and future clinical studies. We intend to report on our clinical plans after further interactions with regulatory authorities.
How is DPX-Survivac being studied as a monotherapy and in combination with immunotherapies?
Schindler: DPX-Survivac generates sustained CD8-positive T cells that target survivin. Survivin is involved in multiple signaling pathways critical for cancer cell survival. Targeting such a critical molecule by immunotherapy makes it difficult for cancer cells to downregulate it without extensively sacrificing their unlimited growth and resistance to standard therapies. Not only has survivin been shown to be highly expressed in ovarian cancer but also many other solid and hematologic malignancies, with limited expression in normal adult tissues, making it an excellent target for cancer immunotherapy. The wide array of cancer types opens the possibility to evaluate our lead candidate in multiple cancer indications as a monotherapy and in combination with immunotherapies or other targeted agents that present complementary anti-cancer mechanisms of action.
We are currently evaluating DPX-Survivac with intermittent low-dose CPA in a phase 2 clinical trial with advanced, recurrent ovarian cancer (DeCidE1 study). We are also conducting an exploratory “basket” clinical trial evaluating DPX-Survivac in combination with pembrolizumab, and intermittent low-dose CPA, in patients with ovarian cancer well as four other cancer indications: non-small cell lung cancer, bladder cancer, microsatellite-instability high cancers and hepatocellular carcinoma).
What is Merck’s role in the IMV clinical trials involving pembrolizumab (Keytruda)?
Schindler: Merck’s pembrolizumab is an immunotherapy known as a checkpoint inhibitor that blocks the PD-1 pathway. This blockade enhances the functional activity of target lymphocytes to facilitate tumor regression and ultimately immune rejection. DPX-Survivac belongs to a novel class of immunotherapy that generates cancer-targeted T cells and has been demonstrated to increase PD-L1 and PD-1 expression in nonclinical studies. Combining these two complementary mechanisms of action is expected to result in an enhanced anti-cancer immune response with the potential to lead to better clinical outcomes.
Merck showed interest in collaboration to evaluate pembrolizumab with DPX-Survivac and intermittent low-dose CPA in the exploratory “basket” trial that explores the combination in multiple solid tumor indications. Additionally, our lead compound is evaluated in combination with pembrolizumab and intermittent low-dose CPA in patients with relapsed, refractory DLBCL in the investigator-initiated SPiReL study.
What is the latest from the SPiReL studies? Are there any updates after IMV presented data at the American Society of Hematology’s annual meeting?
Schindler: The latest clinical update was presented at the annual ASH meeting in a poster presentation by Dr. Neil Berinstein, lead investigator of the SPiReL study and hematologist at the Sunnybrook Health Sciences Center. Dr. Berinstein highlighted that of the seven evaluable patients with higher PD-L1 expression on their tumor cells. Six achieved an objective response suggesting that patients with PD-L1–positive disease may be more likely to respond to the combination of DPX-Survivac with pembrolizumab and intermittent low-dose CPA. We will need to conduct larger efficacy studies, but the early clinical data show that the combination of these two immunotherapies is well tolerated, and an off-the-shelf regimen could be an attractive treatment option.
Any other updates related to IMV’s 2021 plans for DPX-Survivac you would like to share?
Schindler: DPX-Survivac is the first of IMV’s new class of immunotherapy. As explained previously, the wide array of cancer expressing survivin opens the possibility to evaluate our lead candidate in multiple cancer indications as a monotherapy and in combination with immunotherapies or other targeted agents that present complementary anti-cancer mechanisms of action.
The company is currently advancing other cancer immunotherapies using our versatile delivery technology, DPX, to develop new single and multi-targeted agents designed to generate strong, sustained anti-cancer T cell activity.