
Moderna Logo (PRNewsFoto/Moderna Therapeutics)
While the so-called ‘U.K. variant’ of COVID-19 has triggered alarm, it may be a harbinger of things to come, as the novel coronavirus mutates across the world, complicating mass-vaccination efforts.
Moderna (NSDQ:MRNA) detailed its plans to combat the matter in a briefing with investors. While it expects two 100-µg doses of its mRNA-1273 vaccine to be effective against emerging strains of the virus, it fares less well against the South African variant, B.1.351.
The so-called “Brazil variant,” also known as P.1, possesses about 20 mutations. Like the U.K. and South African variants, researchers believe the Brazilian variant to be more transmissible, but it may also be more likely to reinfect people.
“We have followed SARS-CoV-2 mutations since January 2020,” said Moderna CEO Stéphane Bancel in the briefing. The virus has mutated many times, and it will continue to mutate.”
But the mutations that are of “particular concern” are those that involve the SARS-CoV-2 spike protein, said Dr. Stephen Hoge, Moderna president. Variants with such mutations include those from the U.K., South Africa and Brazil. Mutations linked to or near the virus’s receptor-binding domain (RBD) portion of the spike protein (S protein) are especially problematic. “When we develop antibodies that neutralize and protect against the coronavirus, we really find that the antibodies that bind at the top of the spike protein are particularly effective,” said Dr. Stephen Hoge, Moderna president.
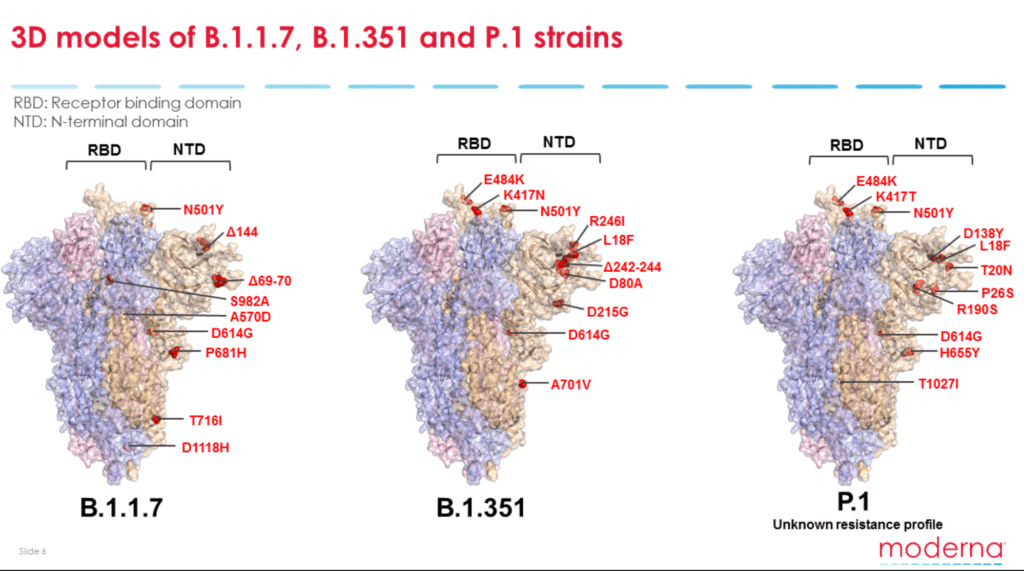
Image courtesy of Moderna
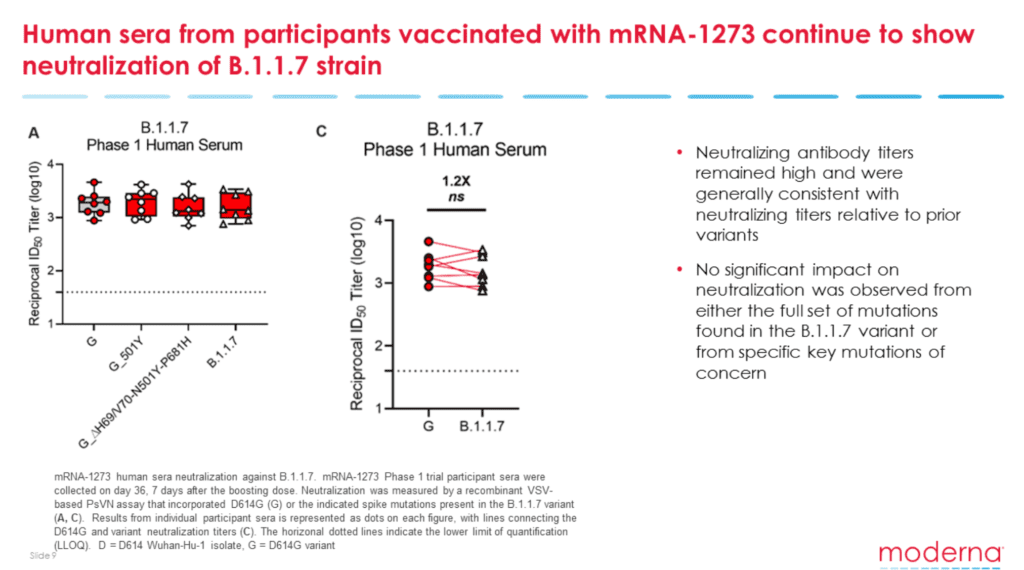
The U.K. variant has a single mutation (N501Y) in the spike protein’s receptor-binding domain. For that reason, both the Moderna and Pfizer-BioNTech seem to be effective against the strain.
But emerging data indicate that the current S-protein-based COVID-19 vaccines may not be as effective against the South African and Brazilian variants as they have two further mutations (E484K and K417N/T) in the receptor-binding domain.
As the virus mutates and its appearance changes compared to the parental strains (D614 and D614G), there is the risk of decreased immunity from vaccination or prior natural infection.
Moderna confirmed using sera from patients vaccinated in a Phase 1 trial that its mRNA-1273 vaccine could stop the replication of the B.1.351 South African variant, but the levels of neutralizing antibodies were 6.4 times lower than the D614G variant.
Vaccine efficacy against COVID-19 variants, including B.1.1.7. Image courtesy of Moderna.
As part of its clinical development strategy, Moderna will test additional booster doses of its mRNA-1273 vaccine to see if they generate enhanced antibody levels to offer further protection against emerging strains.
The company will also test a modified version of its vaccine, mRNA-1273.351, against the variant discovered in South Africa.
“As we continue to follow the story in the weeks, months and perhaps years ahead, it’s important that we remain vigilant and develop potential tools and countermeasures that would allow us to continue to beat back the pandemic,” Hoge said.