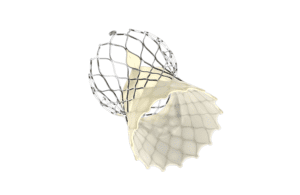
Medtronic (NYSE: MDT) marked the start of the TCT 2022 conference today by announcing the full U.S. launch of its Evolut FX TAVR system.
The medtech giant’s next-generation TAVR won FDA approval in 2021, followed by a limited roll out earlier this year. Edwards Lifesciences —Medtronic’s major U.S. competitor in the transcatheter aortic valve replacement space — announced the launch of its next-gen Sapien 3 Ultra Resilia heart valve earlier in the week.
The Evolut FX system incorporates design changes. For example, it includes gold markers built into the frame to provide implanters with direct visualization of commissure alignment.
There’s also a redesigned catheter tip for a smoother insertion profile. Delivery system flexibility features include an optimized stability layer for a more stable, predictable deployment. As with its Evolut Pro+ predecessor, there are four valve sizes — a large indicated patient treatment range. Medtronic says the Evolut FX has the lowest delivery profile currently on the market.
“This represents a milestone for our structural heart business, as we look to set new expectations for TAVR delivery systems and optimize outcomes for patients. These innovations will equip physicians with improved implant predictability, ultimately improving the overall reliability of the procedure,” said Dr. Jeffrey Popma, VP and chief medical officer for the Structural Heart & Aortic business, which is part of the Cardiovascular Portfolio at Medtronic.
Medtronic at TCT will also unveil finding from four studies that it says will highlight some of the latest clinical insights on TAVR.