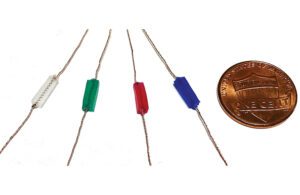
Mechanically active gel-elastomer-nitinol tissue adhesive (MAGENTA) device prototypes made with a nitinol spring and elastomer insulation, with a penny for scale [Photo courtesy of the Wyss Institute at Harvard University]
Harvard bioengineers have created a mechanically active adhesive that can prevent muscle wasting and support atrophy recovery.
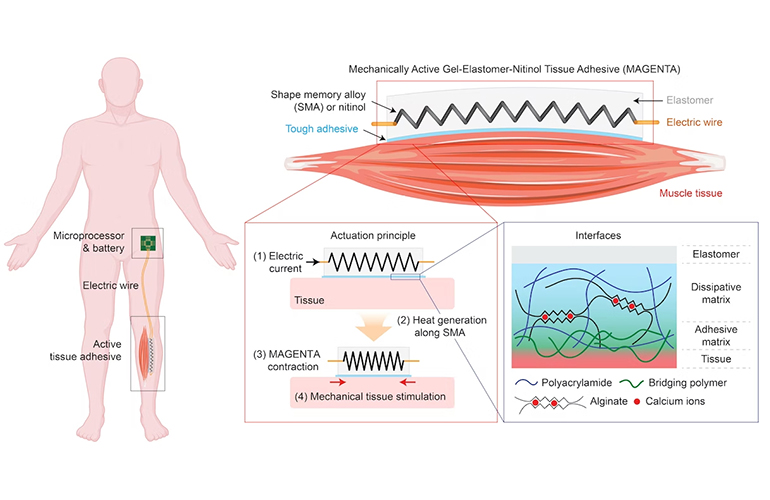
They call it MAGENTA, an acronym for mechanically active gel–elastomer–nitinol tissue adhesive. Researchers from the Wyss Institute for Biologically Inspired Engineering at Harvard University and the Harvard John A. Paulson School of Engineering and Applied Sciences successfully tested MAGENTA in an animal model and published their study in Nature Materials.
“With MAGENTA, we developed a new integrated multi-component system for the mechanostimulation of muscle that can be directly placed on muscle tissue to trigger key molecular pathways for growth,” senior author David Mooney, a Wyss founding core faculty member, said in a news release. “While the study provides first [the] proof-of-concept that externally provided stretching and contraction movements can prevent atrophy in an animal model, we think that the device’s core design can be broadly adapted to various disease settings where atrophy is a major issue.”
Nitinol does it again
The MAGENTA system uses a spring made from nitinol, a nickel-titanium alloy used in medical devices for its shape memory abilities. When heated, the nitinol spring rapidly actuates, controlled by a microprocessor unit programmed with the frequency and duration of stretching and contraction cycles.
An elastomer material insulates the MAGENTA device’s nitinol spring, which is fixed to muscle tissue with a “tough adhesive.” The device transmits the mechanical force deep into the muscle when aligned with the natural axis of muscle movement.
[Illustration courtesy of the Wyss Institute at Harvard University]
The researchers implanted the device on major calf muscles of mice without serious signs of tissue damage or inflammation. The device delivered a mechanical strain of about 15%, which the researchers said matched natural exercise deformation.
The researchers then put the device on mouse legs and put them into a cast for up to two weeks.
“While untreated muscles and muscles treated with the device but not stimulated significantly wasted away during this period, the actively stimulated muscles showed reduced muscle wasting,” first-author and Wyss Technology Development Fellow Sungmin Nam said in the news release. “Our approach could also promote the recovery of muscle mass that already had been lost over a three-week period of immobilization, and induce the activation of the major biochemical mechanotransduction pathways known to elicit protein synthesis and muscle growth.”
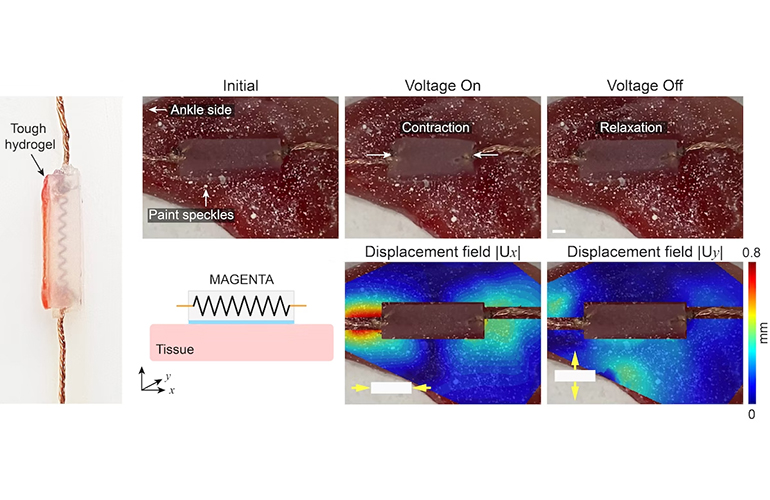
These images show the MAGENTA device, what it looks like when implanted on a mouse calf muscle, and how much displacement the device causes. [Image courtesy of the Wyss Institute at Harvard University]
What’s next for the MAGENTA device?
The researchers also experimented with using light to actuate the device, replacing the wires that connect the nitinol spring to the microprocessor. Laser light shone through the skin was able to actuate the device, but did not achieve the same frequencies, with fat tissue seemingly absorbing some of the light.
The researchers said they believe the device’s performance and sensitivity to light can be improved.
“The general capabilities of MAGENTA and fact that its assembly can be easily scaled from millimeters to several centimeters could make it interesting as a central piece of future mechanotherapy not only to treat atrophy, but perhaps also to accelerate regeneration in the skin, heart and other places that might benefit from this form of mechanotransduction,” Nam said.