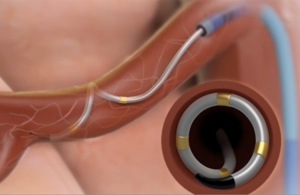
The Symplicity Spyral renal denervation system delivers energy to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
Medtronic (NYSE:MDT) today reported clinically significant and sustained blood pressure reduction among nearly 3,000 people with uncontrolled hypertension treated with the company’s Symplicity renal denervation system.
The study results, reported today out of the 2021 EuroPCR Annual Meeting, come the same week as ReCor Medical touted its own positive renal denervation study at the American College of Cardiology’s 70th Annual Scientific Session.
Together, the new research suggests that renal denervation could be back as a promising medical technology in the cardiovascular space — half a decade after Medtronic announced a major clinical trial had failed to meet its efficacy endpoint. Since the Symplicity HTN-3 study’s failure, Medtronic has sought trial designs that clear up confounding factors such as differing medication regimens and patient compliance.
Renal denervation is a minimally invasive procedure that delivers energy to regulate overactivity of nerves that lead to and from the kidney and help regulate blood pressure control.
The latest results from Medtronic’s prospective, single-arm, global, observational study found a mean reduction of 16.7 mmHg office systolic blood pressure (OSBP) at three years compared to baseline among the nearly 3,000 people with uncontrolled hypertension who were in the study. Health providers treated patients in the study with either the Symplicity renal denervation system utilizing the single-electrode Symplicity catheter or the Symplicity Spyral multi-electrode catheter. Researchers then analyzed outcomes up to three years post-procedure.
Analysis also found a 26% relative risk reduction in major cardiovascular events over three years for the full study cohort treated with renal denervation.
“With this new analysis, we can now help patients continue to see the real-world benefits of renal denervation,” said Dr. Felix Mahfoud, a cardiologist at Saarland University Hospital in Homburg, Germany and the study’s principal investigator.
The next step for Medtronic is the enrollment of an additional 2,000 people with uncontrolled hypertension for the new GSR-Define study. Patients will receive Simplicity Spyral treatment, with data collected on a subgroup out to five years.
“Medtronic’s commitment to creating a minimally invasive solution to treat patients with uncontrolled high blood pressure is evident through our growing body of clinical evidence as part of our Symplicity Global Clinical Program,” said Jason Weidman, SVP and president of Medtronic’s Coronary & Renal Denervation business unit.
The Symplicity Spyral renal denervation system has approval in more than 60 countries globally. Still, it is limited to investigational use in the U.S., Japan and Canada.