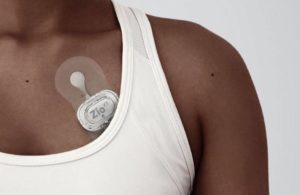
iRhythm’s Zio XT cardiac monitor [Image from iRhythm]
iRhythm Technologies (NSDQ:IRTC) has big plans for 2022 and its remote external electrocardiogram monitors as the medical device developer gears up for growth.
President and CEO Quentin Blackford is looking to hire an international general manager among other roles in the coming months, he told Medical Design & Outsourcing after presenting at this month’s 2022 J.P. Morgan Healthcare Conference, where he laid out his vision for profitably scaling the company.
Asked by MDO whether Blackford is planning a reorganization or restructuring after he discussed the cost-consciousness side of his growth plans, an external spokesperson offered more details from the CEO.
“At a leadership level, we are actively structuring our C-suite in order to create long-term sustainable growth,” Blackford said. “The goal is to enable iRhythm to be a global organization that creates significant value for both the healthcare system and our investors. We’ve recently appointed some talented industry veterans to COO, CCO and GC, and expect to hire additional roles over the next couple of months, such as an international GM.”
Blackford also previewed plans to roll out asymptomatic atrial fibrillation (AFib) screening pilots with payors in 2022. Screening for “silent” AFib can detect problems before an event like a stroke or heart failure, giving doctors more time for treatment at a lower cost.
AFib in the U.S. is associated with approximately 450,000 hospitalizations per year and 158,000 deaths per year.

iRhythm President and CEO Quentin Blackford [Photo courtesy of iRhythm]
Symptomatic patients undergo about 5.5 million ambulatory cardiac monitoring tests for arrhythmias in the U.S. each year, and around 5 million such tests in international markets that San Francisco-based iRhythm is targeting for growth, such as Japan and the U.K.
Blackford said he sees “tremendous opportunity” in screening for silent AFib, saying there are more than 10 million people in the U.S. and at least that many abroad with conditions placing them at high risk for Afib.
“They have no idea that they have undiagnosed arrhythmias that we believe we ought to be screening for at some frequency,” Blackford said in his presentation, noting other potential opportunities such as predicting strokes, heart failure, hypertension and sleep apnea. AFib increases the risk of stroke by five times, for example.

iRhythm’s Zio XT cardiac monitoring device [Image courtesy of iRhythm]
The pilots launching in 2022 will use Zio XT devices to screen patients with conditions that lead to a high prevalence of undetected AFib or other arrhythmias. Blackford said iRhythm’s pilots are generally targeting insurance companies with the goal of demonstrating that the screenings can save costs — and patients — in the long run, but he said it’s too early to name any of the payors.
While the pilots will launch with the Zio XT patch, iRhythm plans to begin market evaluation of its new Zio Watch in late 2022 or early 2023 and bring it into the pilots once approved.
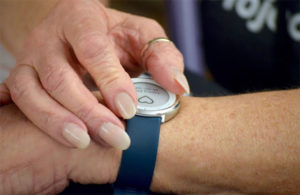
Verily’s Study Watch will serve as iRhythm’s Zio Watch. [Photo courtesy of Verily]
Designed through a partnership with Verily — a subsidiary of Google parent company Alphabet (NASDAQ:GOOGL) — the Zio Watch is more clinically focused on AFib than consumer options like the Apple Watch. iRhythm says its EKG data repository has more than 1 billion hours of curated data from more than 4 million patients.
“Our artificial intelligence capability and deep learning algorithms are a significant differentiator” from competitors, Blackford said. “Not only are we monitoring heartbeats, but we’re listening for beat types, beat rates and rhythms, all of which our algorithms are bringing together in their interpretations.”