The quality analysis tools driving speed to market are now built right into the control system.
It is more important than ever for pharmaceutical manufacturers to deliver treatments quickly and cost-effectively around the globe. With the world’s new focus on rapid results, gone are the days when product could sit in work-in-progress (WIP) for days, weeks, or even months, waiting on lab testing for quality validation.
Plants are putting massive effort into digitally transforming operations to move toward level five of BioPhorum’s digital plant maturity model. As they move up the model, organizations realize lean manufacturing benefits while more quickly and safely putting critical treatments in the hands of patients in need (Figure 1).
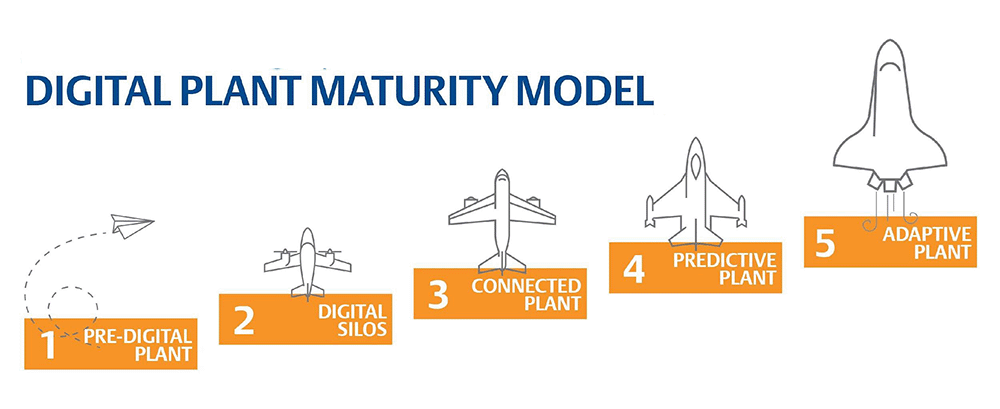
Figure 1. BioPhorum’s digital plant maturity model helps IT and OT digitally transform life sciences manufacturing. Image courtesy of Emerson.
Implementing in-line, real-time, continuous, closed-loop process verification and control with the automated real-time quality release is a critical step toward optimizing facility performance and throughput. Process analytical technology (PAT), which relies heavily on incorporating multivariate spectral analysis data, is an important part of this process. But until recently, PAT required a massive undertaking of time, resources and targeted expertise. Moreover, even after organizations committed those resources, they typically ended up with complex, fragile systems requiring significant upkeep over the lifecycle.
But today, more manufacturers are implementing closed-loop control with a new digital transformation strategy to meet FDA guidance and drive toward autonomous production and increased speed to market. They achieve increased stability and performance by bringing spectral waveform data directly into the control system.
The traditional, layered approach to PAT
Ensuring treatments are ready for patient use always necessitates running tests requiring complex multivariate data. Unfortunately, many of these tests — glucose levels, cell density, concentration of active ingredient, pH, dissolved oxygen and many more — are run manually today. The process involves the following:
- Taking a sample.
- Delivering it to the lab.
- Running tests.
- Waiting for results.
- Validating those results.
- Approving the batch.
Each step requires time and personnel and introduces the risk of human error.
Moreover, additional quality tests are often performed after manufacturing is complete, delaying release and making it impossible to recover from deviations. With batches valued in the thousands or even millions of dollars, the financial impact can be significant. Even in cases where testing is performed during manufacturing, the time delay can make it difficult to save an off-spec batch.
Forward-thinking organizations eliminate manual testing by implementing PAT in real-time to bring spectral analyzers in line with the process. These analyzers collect a wide spectrum of data — approximately 3,000 to 4,000 values — every second or so.
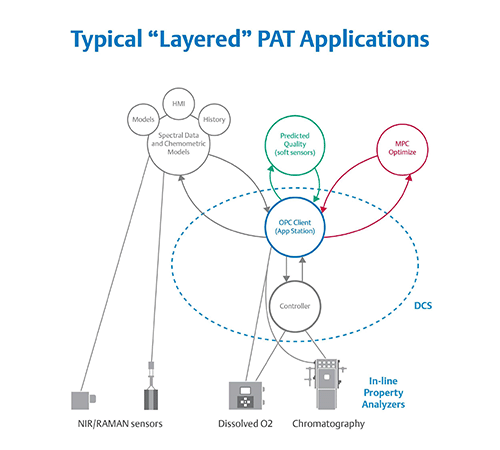
Figure 2. The layered approach to PAT has multiple user interfaces and is difficult to implement, maintain,
and validate. Image courtesy of Emerson.
Historically, turning three or four thousand pieces of spectral data into a univariate value that is useful for the control system has required a layered approach. First, an engineer developed a model offline to be validated as part of the process. The model was then run on computers at level three of the Purdue model to translate analyzer data into a value useful for control. Finally, that value was passed to the controllers on level two to adjust the process in real-time (Figure 2).
While the layered model of PAT enables closed-loop control, the connections among systems are typically quite fragile. To support such a model, operators require three or four disparate systems to communicate seamlessly. The analyzer must talk to the third-party system performing the conversion, which, in turn, communicates with a PAT application comparing the model and values, which itself must share data with the control system.
This configuration creates a complex web for IT and OT to implement and maintain. It also creates a wide surface area for compatibility problems because changing any one element risks breaking something else. Moreover, validating all these systems is very complex and time-consuming.
Integration reduces complexity

Figure 3. Embedded PAT, like that provided with Emerson’s DeltaV Spectral PAT, provides a robust architecture that is easy to implement and maintain. Working in a single, integrated environment makes the system more secure and easier to validate. Image courtesy of Emerson.
To reduce complexity and design more stable closed-loop process control, organizations are collapsing the traditional architecture with integrated PAT. Using PAT embedded in the control system, operations teams bring spectral signals directly into control system blocks via OPC UA (Figure 3).
The control system then performs chemometric model calculations within the real-time control model execution. A PAT model function block in the control system processes and accesses spectral array signals directly to perform quality attribute calculations for real-time monitoring (Figure 4).
Integrated PAT helps plants move toward fast, fully-automated execution without human participation, and it also helps build a foundation for better regulatory management. In the most advanced systems, change control mechanisms help confirm processes are using the right model version, simplifying audits. In addition, some systems also perform real-time diagnostics to confirm analyzers are working properly while simultaneously creating comprehensive logs designed for offline comparison against the model to further ensure and document quality.
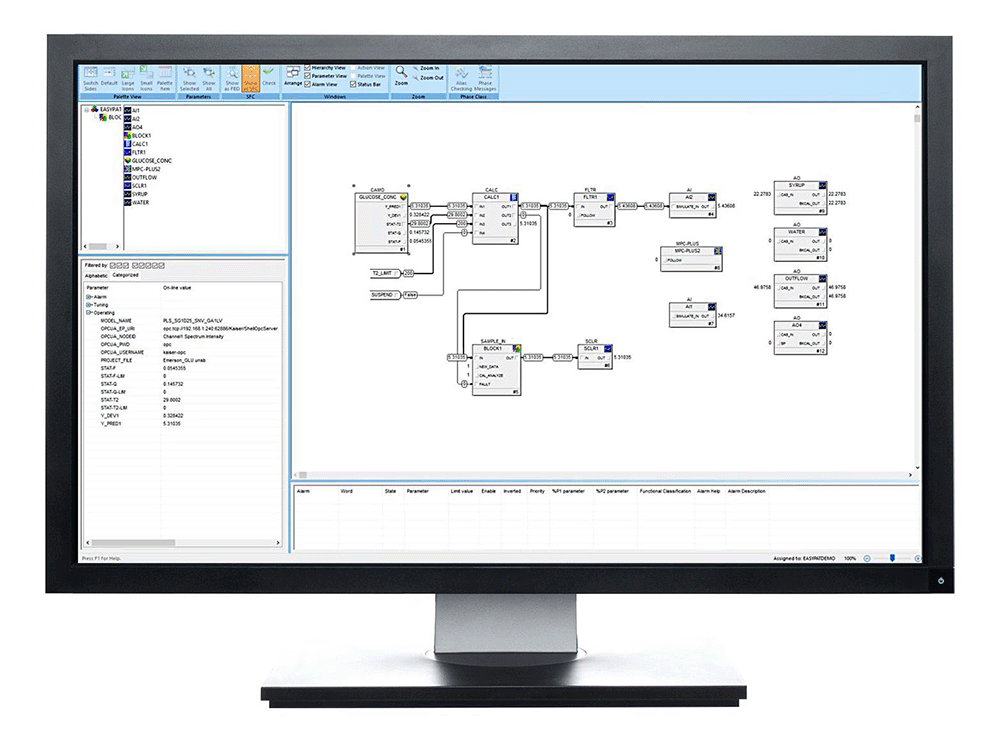
Figure 4. With embedded PAT, spectral array signals are sent directly to a function block in the control system. Image courtesy of Emerson.
Preparing for closed-loop control
As operations teams prepare to implement integrated PAT to unlock advanced closed-loop control, there are concrete steps they can take to help ease the transition. For example, performing a process audit can help build the case for in-line analysis and identify return on investment (ROI) to support a PAT project.
Typical manufacturing lines often have four to six units that could benefit from in-line analytical measurement for closed-loop process control. Identifying the time savings of integrated PAT versus the traditional process — sample, then wait, then act — coupled with the impact of reducing WIP builds a strong case for integrated PAT.
Though the complexity of PAT systems can be a point of concern, advanced integrated PAT systems have built-in solutions to show and document safe, accurate, repeatable processes. In addition, embedded technologies perform analytics to validate proper analyzer operation, and management of change tools verify that a plant is using the correct model version for a given batch. Demonstrating these technologies can give regulatory groups the confidence they need to support this new approach to improve manufacturing performance.
Faster release means faster ROI
In the most complex production scenarios, life sciences manufacturers can find themselves sitting on millions or even billions of dollars in WIP inventory while waiting on quality test results. As with any emerging paradigm shift, the technologies to unlock fully-automated manufacturing and eliminate WIP altogether are progressing rapidly. The shift is happening fast, and looking toward a fully-automated future will help organizations secure speed-to-market and competitive advantage in the decades to come.
The foundational technologies available today to prepare for such a future bring fiscal, safety and quality benefits of their own. With the shift toward integrated design, unlocking the benefits of PAT has never been easier.
Taking advantage of integrated PAT can deliver rapid ROI today while simultaneously preparing your plant for the automated strategies that will dominate the future of life sciences manufacturing.

Bob Lenich
About the Authors
Bob Lenich is director of global life sciences at Emerson Automation Solutions. He is a life-long learner who stays engaged in new technology and organizational trends. In his 40 years in the industry, Bob continually aims to solve operating issues across the process industries to help life science manufacturing improve people’s lives.
Bob has a Bachelor of Science in chemical engineering from Rose Hulman Institute of Technology and a Master of Business Administration degree from the University of Texas.

Bruce Greenwald
Bruce Greenwald is the DeltaV Platform Business Development Manager for Emerson Automation Solutions. In his current role, Bruce assists customers in understanding the features and benefits of DeltaV to improve their automation experience.
He is a 1979 graduate of the University of Kansas with a Bachelor of Science degree in chemical engineering.
Bruce started his career with Dow Chemical in Freeport, Texas and joined Fisher Controls in 1983 as a systems engineer. He joined the RE Mason Company, an Emerson Impact Partner, in 2000, executing PROVOX and DeltaV projects. Bruce held several positions at RE Mason, and in 2011, rejoined Emerson. His four-decade-long career has been focused on process control.