
[Image courtesy of Chad Davis via Flickr]
Where would clinical trial diversity be without the COVID-19 pandemic and the death of George Floyd on May 25, 2020? It likely wouldn’t be the priority it is today across the industry, according to Ariel Katz, CEO and co-founder of H1, a New York–based healthcare and data analytics platform firm.
After the death of George Floyd, scores of pharma companies created or beefed up departments related to diversity and inclusion. “Basically, every pharma company and biotech is now doing something around diversity and inclusion and social determinants of health,” Katz said.
On Google, interest in the search phrase ‘clinical trial diversity’ surged from July 2020 to July 2021. Interest in the phrase continues to remain strong but has dipped from its peak.
Things have changed considerably during the pandemic.
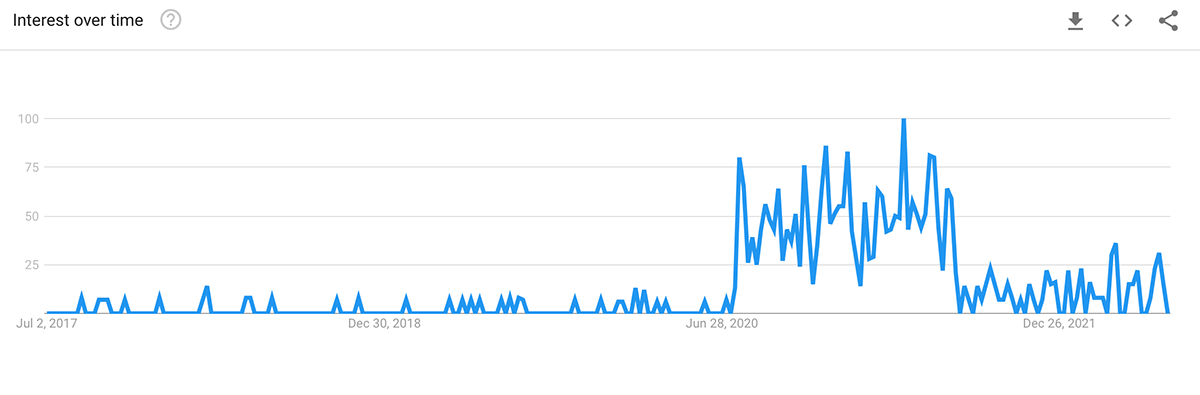
[Search volume for ‘clinical trial diversity’ via Google Search Trends]
An overview of six early COVID-19 trials found that one-third didn’t report race/ethnicity information. In the remaining four, Black patients were “underrepresented,” concluded the analysis published in ScienceDirect.
The most dangerous phase of the pandemic also disproportionately affected racial and ethnic minority groups, who faced an elevated risk of getting sick and dying, as the CDC noted.
In October 2020, COVID-19 vaccine maker Moderna announced that it had temporarily halted its first Phase 3 clinical trial to improve the recruitment of diverse populations.
A 2021 JAMA article surveying 230 U.S.-based vaccine clinical trials concluded that “Black or African American, American Indian or Alaska Native, Hispanic or Latino, and older adults were underrepresented.”
FDA data for 2020 paints a similar picture. That year, three-quarters of clinical trial participants were white when only 61.6% of the overall population was. Conversely, 8% of clinical trial participants that year were Black, while 13.4% of the U.S. population is.
Things are beginning to change. “I think COVID shone too much of a bright light on the lack of diversity in clinical trials for us not to make progress,” said Otis Johnson, chief diversity, inclusion and sustainability officer at Clario, in an interview last year.
The pandemic and the death of George Floyd made clinical trial diversity a prominent issue in public discourse. “It was all over the news,” Katz recalled.
Until recently, however, few pharma companies had mature tools to optimize diversity.
H1 responded to the surge in demand from its pharma clients by adding new diversity tools to its database. Now, if a drug company using the platform searches for doctors specializing in, say, bladder cancer, they can find the race/ethnicity and gender of an individual doctor’s patients. “Now, if you want to find someone who could run a clinical trial with diverse patient populations, you know exactly how to find them,” Katz said.
Earlier this year, FDA released new draft guidance describing plans for increased diversity in U.S. clinical studies.
Clinical trial diversity is “early in evolution,” Katz said, but companies are now “politically forced” to focus on it.