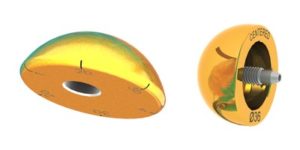
TiN (titanium nitride)-coated humeral heads and glenospheres (Image courtesy of FX Shoulder USA)
FX Shoulder USA announced today that the FDA issued 510(k) clearance for a new version of its humeral head and glenosphere shoulder implants.
The Dallas-based company said it will now offer implants coated in TiN (titanium nitrate), a material that some studies indicate will increase surface hardness and reduce wear. The FDA-cleared devices will be identical to the company’s existing humeral heads and glenospheres except for the coating.
“We are now able to reach a broad market and provide surgeons even more shoulder arthroplasty solutions to address patients’ needs,” said Baptiste Martin, CEO of FX Shoulder USA, in a press release. “As we continue to expand our portfolio, we truly have one of the more comprehensive portfolios dedicated exclusively to shoulder arthroplasty.”
The TiN coated humeral head is compatible with the company’s Humelock II and Humeris shoulder in the anatomic construct while the TiN coated glenosphere is compatible with the Humelock II.
Earlier this year, FX Shoulder USA secured 510(k) clearance for its Stability humeral cup and 135/145° Stability humeral cup. The new stability cups are part of a shoulder arthroplasty portfolio and provide an option to U.S. surgeons for primary, trauma or revision reverse shoulder arthroplasty procedures.
FX Shoulder USA, Inc. is is the direct provider of FX Solutions shoulder replacement devices in the U.S. FX Shoulder USA, Inc., founded in January 2018, focuses exclusively on shoulder arthroplasty.
The company initially purchased its shoulder line from Small Bone Innovations in 2010.