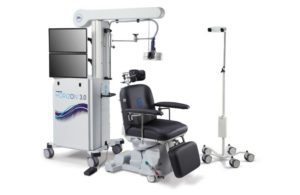
[Image from Magstim]
Magstim announced today that it received FDA 510(k) clearance for its Horizon 3.0 transcranial magnetic stimulation platform.
Horizon 3.0 offers enhanced workflows with navigated transcranial magnetic stimulation (TMS) and analytics for the clinical setting, offering connected care at networked sites, according to a news release.