It’s difficult for a company to achieve excellence when batches are lost and maintenance crews must fight fires due to equipment failure. Still, solutions are available now to address these and other issues in the pharmaceutical industry.
Most life sciences plants and facilities face many issues impacting reliability, throughput, and uptime. For example, a bioreactor’s agitator slows down during a batch process without the operator realizing it, and the result is a quarantined batch and production schedule delay while it’s addressed.
In another common scenario, a unit’s control system says the pH of a given batch has moved out of its tolerance range, but plant personnel cannot quickly determine if it is due to a process upset or a malfunctioning sensor. Grab samples must be analyzed in the lab to verify, delaying production.
Or a pump used to empty a bioreactor at a critical time is malfunctioning. It may be a problem with the variable frequency drive, motor, or pump — but in either case, the result is reduced yield for the batch due to extended reaction time.
The common denominator of all three of these issues is poor equipment reliability. Related problems can take many forms:
- Outright equipment failure or poor performance can spoil batches or shut down production completely.
- Inability to determine equipment condition results in inconsistent maintenance, perpetuating inconsistent performance.
- Inconsistency of manual condition analysis and data collection impedes effective diagnostics.
So, how does a plant overcome these problems when each is an obstacle to maximizing production and overall profitability? Can reliability be quantified and measured like temperature or pressure and then improved?
Achieving operational integrity
There are many ways to define operational integrity within life sciences. Still, most include concepts related to product quality, predictable and controlled processes, reliable equipment performance, consistent production availability, and so forth. For the balance of this article, we’ll concentrate on reliable equipment performance because this element is a building block for all the others.
For the sake of contrast, what is the absence of reliable equipment performance? Unplanned asset failures, suboptimal asset performance, poor maintenance planning, and untimely follow through — all of which cause shutdowns, under-utilized production equipment, deviations, and material/product losses.
How does this happen? Even within the life sciences industry, many companies and individual plants operate in a run-to-failure mode and often experience equipment failures during a production batch. They typically do not monitor equipment condition, so they go unrecognized even with signs of impending failure. This philosophy disrupts schedules and results in costly failures, so maintenance expenditures are excessive.
The opposite extreme is an over-maintained plant, where maintenance actions are dictated by a rigorous schedule, regardless of equipment condition. This likely results in fewer unscheduled outages but with high operating costs and lower availability because inordinate amounts of time are spent performing unnecessary maintenance.
The underlying cause of both extremes is a lack of information and resulting corrective action. So let’s return to the three situations mentioned at the beginning of the article and dig deeper.
What do failures indicate?
The agitator could have slowed because of worn gears, an internal part fracturing, dry bearings, or some other cause. On the other hand, the operator might have no clear vantage point or might not pay close enough attention. In either case, leaving this critical verification to human observation isn’t a good idea.
The traditional method to address issues like this is for the control system running the process to detect the problem by monitoring the agitator’s current draw. When operating correctly in this process, the motor should draw, say 5 A. If it falls to 0 A, obviously there is a power problem. However, if it begins to creep up, perhaps over several batches, the motor must work harder to overcome resistance, which means a problem is developing. The signs of nascent failure are there, provided they are monitored and acted upon.
The pH sensor is representative of all process instrumentation used in manufacturing. Instruments and sensors must be monitored constantly and re-calibrated on appropriate cycles to ensure manufacturing processes are within defined process windows. This is especially important during long production runs with the potential to drift mid-run.
Where a facility depends on conventional instrumentation, this approach calls for a major internal program for calibration management (Figure 1). The more practical approach is to select sensors and instruments with high stability for the most critical process parameters. This consideration should be top-of-list for a company wanting to reduce costs and time spent to re-calibrate instrumentation.
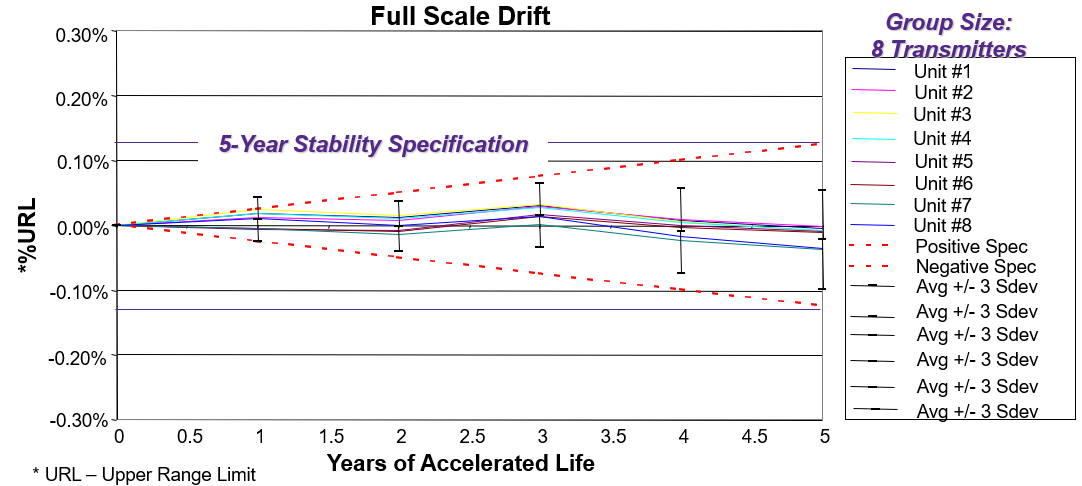
Figure 1: Process instruments and sensors must remain in calibration, requiring a high degree of stability. Figure courtesy of Emerson.
Heavy rotating equipment installations, primarily pumps and air handling units, are often left to run until failure or until a problem has advanced to the point that it is obvious to operators. But by this time, the abnormal condition is probably impacting production, or it will very soon. Trying to decide when something needs attention is typically a manual activity performed by a maintenance technician during rounds. If there isn’t an obvious problem, the technician leaves things as-is until the next scheduled check, often missing a problem that will flare up before the next check.
Adding vibration monitoring sensors (Figure 2) with wireless connectivity options and asset-monitoring software provides a comprehensive solution to avoid critical rotating failures.
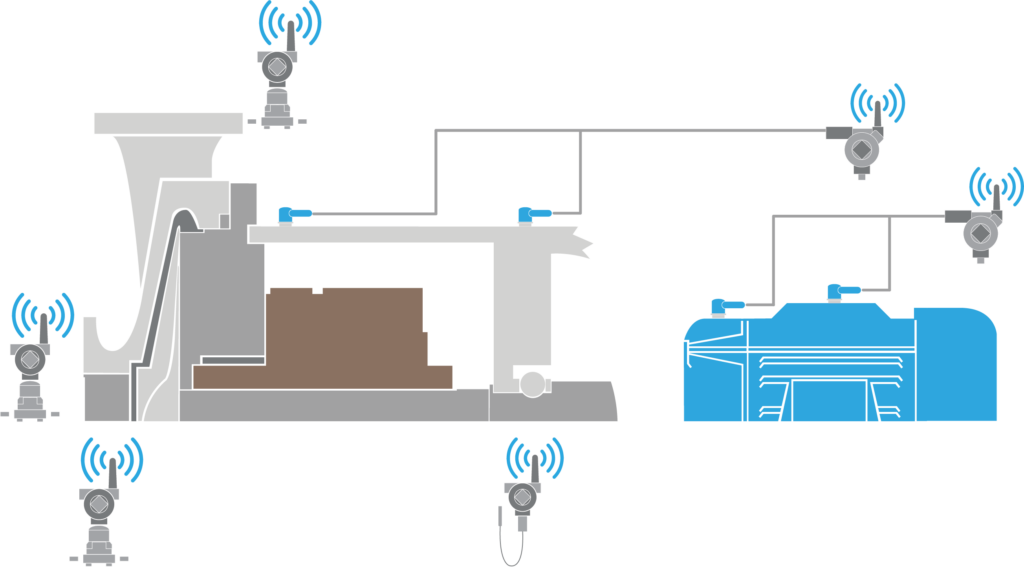
Figure 2: Wireless sensors added to rotating equipment, such as pumps, can detect problems with bearings or alignment long before an outage. Figure courtesy of Emerson.
Other equipment health measurements can also be monitored and analyzed by machinery health monitoring software (Figure 3). This software tracks equipment performance in real-time to detect potential issues, and it provides diagnostics and analysis to help plant personnel avoid production performance problems.
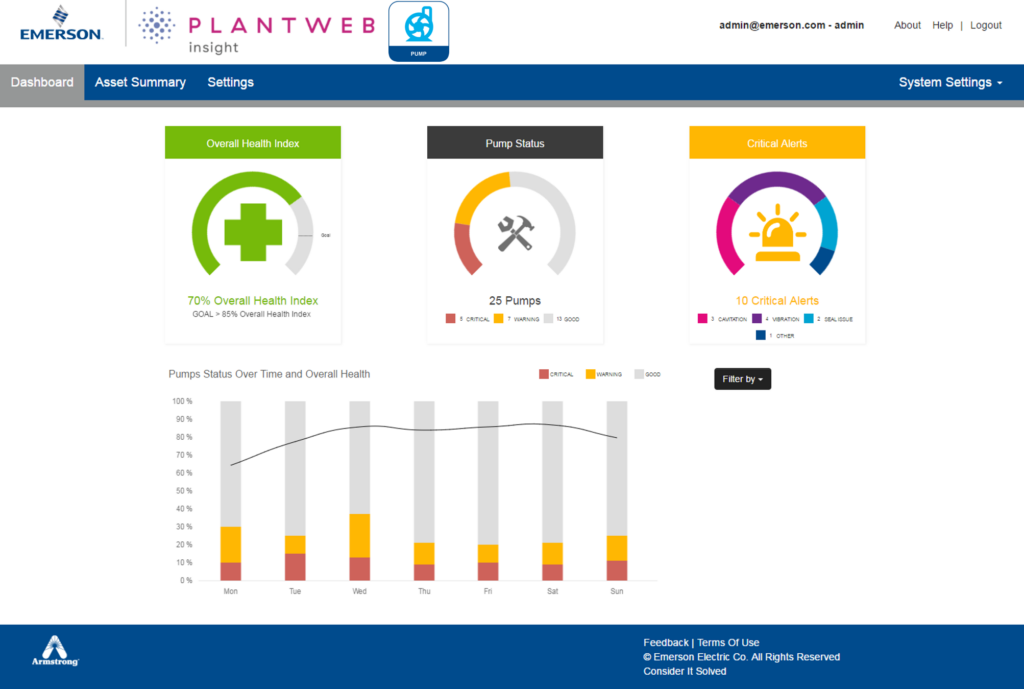
Figure 3: Machinery health monitoring software, such as Emerson’s Plantweb™ Insight, captures and historizes diagnostic data and then presents it to operators in easy-to-understand dashboards. Figure courtesy of Emerson.
Diagnostic data, collected from the type of sensors and methods just discussed, combined with forensic examination of failed devices, support failure modes and effects analysis (FMEA) to facilitate changes in practices, which can ward off the subsequent unplanned failure (Figure 4). For example, if a bearing failed due to poor lubrication, looking at how to change maintenance procedures is a prominent part of the ongoing solution.
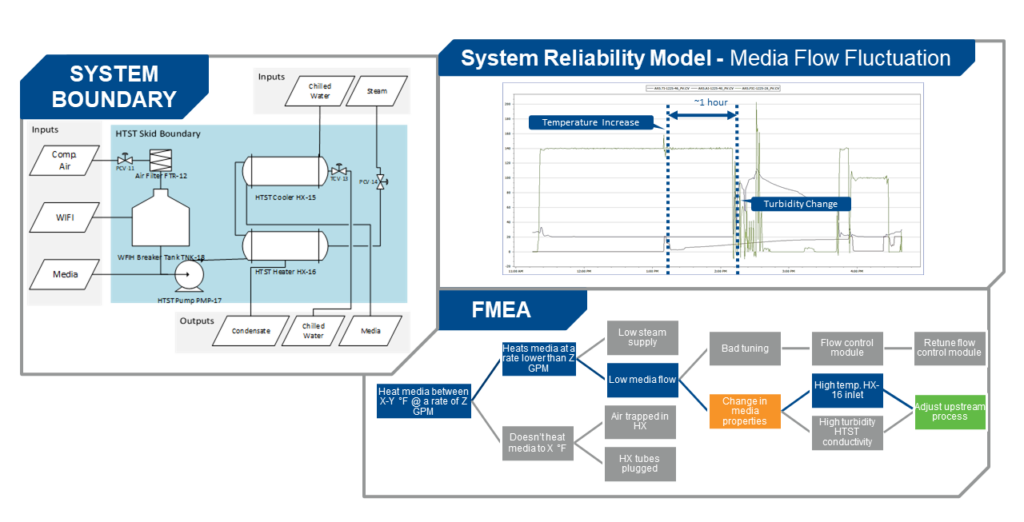
Figure 4: Failure modes and effect analysis can use real-time sensor monitoring data to predict failures. Figure courtesy of Emerson.
Machinery health monitoring software is an integral part of FMEA since it can provide analysis based on historical data collected from a wide range of condition monitoring sensors. As a result, it becomes a critical tool for changing methods and practices for improved operational integrity. Today’s platforms can also use artificial intelligence (AI) techniques to analyze data to find trends and causes that would evade most human evaluators.
What, and then when
So far, the discussion has been about detecting problems, but when is the best time to deal with them? Ideally, maintenance operations should be carried out when the equipment is not used to avoid impacting production. This sounds obvious, but real-world observations show it’s easier said than done. Production planning, scheduling, and maintenance departments don’t always communicate as they should, resulting in equipment unavailability or launching a production run with an asset on the brink of failure.
A real-time modeling system (RTMS) allows all functional areas to visualize facility constraints, maximize production, schedule events, and understand the implications of any change in the manufacturing process (Figure 5). By collecting data from across the organization, an RTMS can consolidate, analyze, and present an accurate facility model, its processes, and schedules. The model produces an optimal scheduling overview and debottlenecking and capacity analyses for process optimization.
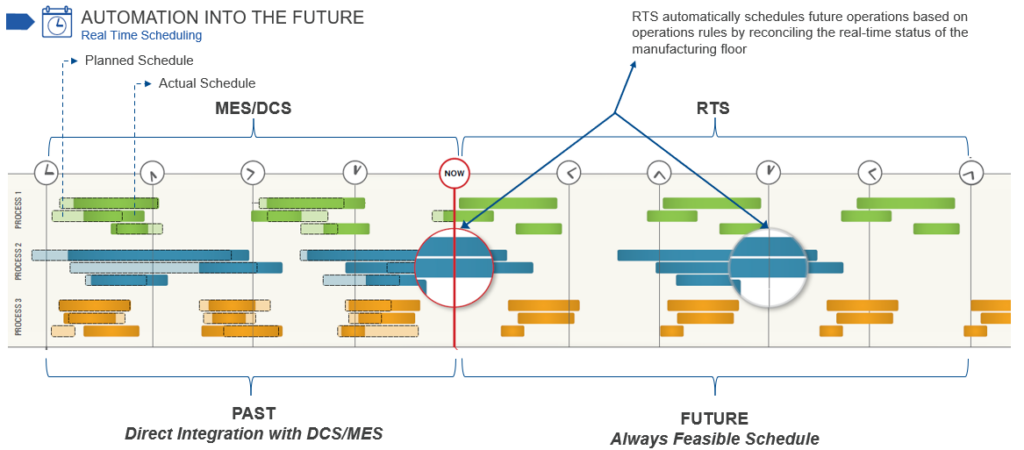
Figure 5: A real-time modeling system makes it easier for all functional areas, including maintenance, to optimize actions, avoiding production complications. Figure courtesy of Emerson.
Maintenance can review the plans and look for ‘white spaces’ in the schedule where specific units and pieces of equipment will not be in use. Coupled with asset monitoring data, it is possible to determine what maintenance actions will be necessary by when and then slot these activities into the available gaps. This ensures effective maintenance scheduling and avoids production disruptions.
Real-world results
One North American-based biopharmaceutical company challenged its maintenance and production teams to reduce costs and cycle times while dealing with local labor shortages. Specifically, they needed to spend less time struggling with manual maintenance planning and find a means to reduce downtime during column packing operations. Too frequently, production was delayed because a critical instrument or other piece of equipment was being calibrated or repaired.
After adopting a real-time scheduling system, the plant found it could do a much better job of planning maintenance. Automating the planning process reduced time spent by 95% compared to manual efforts. In addition, it ensured all critical equipment was available and ready to go when production plans called for it. Working more efficiently on these routine tasks made it easier to work through the short-handed staffing situation and even put resources to work on improving column packing procedures.
Another North American-based life sciences manufacturer struggled with production interruptions due to unplanned downtime. The facility had identified a group of critical assets, including a variety of pumps, but had little or no condition data for many of them, forcing maintenance to scramble if outages were to be avoided.
After deploying a machinery health monitoring software platform with AI analysis capabilities, the maintenance team identified several standard failure modes for their rotating equipment. Then, after consulting with an expert, the maintenance team used the new information to extend the monitoring system and analysis tools. This solved the problem of bad-actor assets and increased the number of production batches, free from interruptions.
A northeast U.S. life sciences manufacturer was facing a wave of retirements that would reduce its maintenance staff by 25%, including many of the most experienced individuals. The company also planned to change the slate of products made at the facility to a group of lower-margin biosimilars. This would tighten budgets on all sides, resulting in a smaller workforce permanently. In addition, the facility had been using time-based maintenance scheduling, but it was hoping to change to failure detection techniques to reduce required maintenance activities and overall costs.
It deployed a range of monitoring sensors on critical assets and then selected a machinery health monitoring software platform equipped with AI to help detect nascent failures. This provided much higher visibility into asset health so that the facility could improve production, even with a smaller staff. After a year of operation with the new system, management calculated the new systems brought more than a 600% return on the investment.
Using the right tools
The life sciences industry is evolving. Changing from batch manufacturing to continuous; accelerating technology transfer to speed new therapies to market; and optimizing unit and facility throughput are great examples of this strategic evolution. Achieving operational integrity is a critical component of this process, and it is necessary for optimal operations.
Given the wide range of basic and highly sophisticated tools available today, there is no reason a company in any industry, especially life sciences, cannot improve operational integrity. The techniques are proven, and countless companies have put them to work and realized benefits.

Bob Lenich
About the Author
Bob Lenich is director of global life sciences at Emerson Automation Solutions. He is a life-long learner who stays engaged in new technology and organizational trends. In his 40 years in the industry, Bob continually aims to solve operating issues across the process industries to help life science manufacturing improve people’s lives. Bob has a bachelor of science in chemical engineering from Rose Hulman Institute of Technology and an MBA degree from the University of Texas.