A COVID-19 immunity detection test developed by MIT could help people determine their risk of infection. [Image courtesy of MIT]
MIT researchers have developed a device for predicting an individual’s COVID-19 immunity and are looking for a diagnostic company to get it manufactured in large numbers and approved by the FDA.
The lateral flow test uses the same technology as at-home rapid antigen COVID-19 tests to measure neutralizing antibodies for SARS-CoV-2 in a blood sample, the researchers said in a study published in Cell Reports Methods.
The researchers have filed for a patent on the technology, which could help people weigh their COVID-19 immunity against risk and determine necessary precautions such as boosters.
RELATED: Harvard researchers plan to sell at-home, PCR-grade COVID testing system
The development comes more than two years into the pandemic as the latest virus mutations show increasing evasion to immunity from vaccines and past infections, and leading antibody treatments have lost effectiveness against the omicron varient.

Dr. Hojun Li is the the Charles W. and Jennifer C. Johnson clinical investigator at MIT’s Koch Institute for Integrative Cancer Research and a pediatrician at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. [Photo courtesy of MIT]
“Among the general population, many people probably want to know how well protected they are,” study senior author Dr. Hojun Li said in a news release. “But I think where this test might make the biggest difference is for anybody who is receiving chemotherapy, anybody who’s on immunosuppressive drugs for rheumatologic disorders or autoimmune diseases, and for anybody who’s elderly or doesn’t mount good immune responses in general. These are all people who might need to be boosted sooner or receive more doses to achieve adequate protection.”
The researchers said they designed the COVID-19 immunity test to be able to swap in different viral spike proteins to detect existing and future SARS-CoV-2 variants. Drawing inspiration from at-home pregnancy tests back when the pandemic began, Li’s lab developed a device to detect antibodies that block the SARS-CoV-2 receptor binding domain (RBD) from binding to the ACE2 human receptor that the virus uses to infect cells.
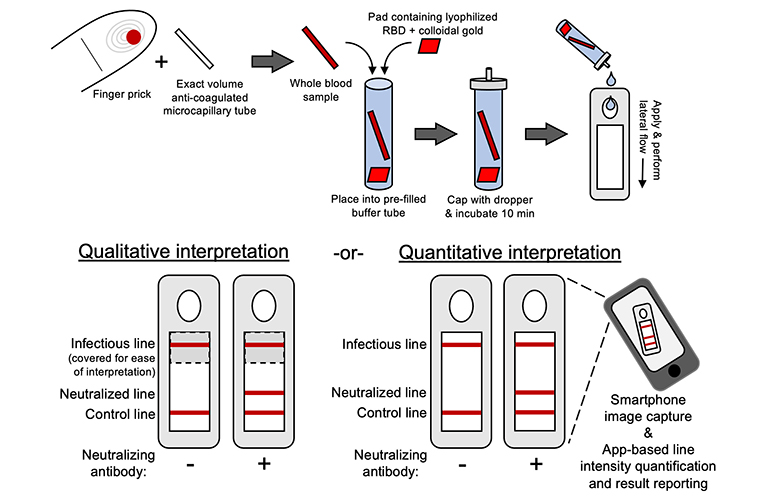
Here’s how the COVID-19 immunity detection device works, according to MIT:
“The first step in the test is to mix human blood samples with viral RBD protein that has been labeled with tiny gold particles that can be visualized when bound to a paper strip. After allowing time for antibodies in the sample to interact with the viral protein, a few drops of the sample are placed on a test strip embedded with two test lines. One of these lines attracts free viral RBD proteins, while the other attracts any RBD that has been captured by neutralizing antibodies. A strong signal from the second line indicates a high level of neutralizing antibodies in the sample. There is also a control line that detects free gold particles, confirming that the solution flowed across the entire strip.”
The COVID-19 immunity testing kit includes a testing cartridge with a paper test strip and a finger prick lancet to obtain less than 10 microliters of blood. After mixing the sample with reagents and waiting 10 minutes, the sample is placed in the test cartridge, and results are revealed in another 10 minutes.
A user can look at the lines to see whether antibodies were detected, while a smartphone app measures the intensity of each line to calculate the levels of neutralized RBD protein versus infectious RBD protein. More neutralizing antibody in the blood could mean a great COVID-19 immunity and lower risk of infection.
[Image courtesy of MIT]