A top Abbott executive explains how the company’s FlexNav delivery system could make the Portico TAVR competitive.
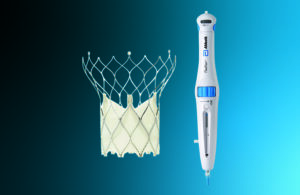
The Portico with FlexNav TAVR system [Image courtesy of Abbott]
Abbott is aiming to claw away market share from transcatheter aortic valve replacement pioneers Edwards Lifesciences and Medtronic.
The Abbott Park, Illinois–based medtech company in September announced FDA approval of its Portico with FlexNav TAVR system, more than a year after it secured CE Mark approval in Europe. For now, the approval covers symptomatic, severe aortic stenosis associated with a high or extreme risk for open-heart surgery.
The Portico valve boasts a self-expanding design with intra-annular (within the native valve) leaflets. The Portico’s creators designed its structure to provide optimal blood flow when placed inside a patient’s natural valve. It also preserves access to the critical coronary arteries for future interventions.
But Santosh Prabhu, the division VP of Abbott Structural Heart, says the FlexNav delivery system is the area where the real ingenuity lies.
“I think it’s a major innovation in how the valve is delivered, based on the innovation that has gone into the design of that delivery system,” Prabhu said during an interview that originally aired in November on our DeviceTalks Weekly podcast.
Abbott wanted to create a TAVR delivery system that was flexible, safe, able to get through tough anatomies, and easy to use.
“The smoother the delivery is, the better it is. The more accurately the doctor is able to place the valve, the better outcomes he gets, the better for the patients,” Prabhu said.