Transmission electron micrograph of two Adenovirus particles. Image courtesy of Wikipedia.
After regulatory authorities in the U.S. and Europe have either paused or constrained the use of COVID-19 vaccines from AstraZeneca (LON:AZN) and Johnson & Johnson (NYSE:JNJ), some regulatory authorities wonder why the adenoviral-vector vaccines have been potentially linked to similar blood clotting events.
Researchers have observed blood clotting events associated with adenoviral vectors since the late-1990s in early experiments with the platform. But the safety of the platform has improved as genetic engineering has advanced.
The history of using viruses as vectors dates back to the mid-1970s when scientists Dr. J. Michael Bishop and Dr. Harold Varmus demonstrated that gamma-retroviruses could acquire cellular genes.
Researchers began creating adenoviral vectors in the 1980s, testing replication-defective Ad vectors at that time. Most focused on adenovirus type 5 (Ad5).
Scientists believed that Ad5 held a particular promise for gene therapy to correct rare genetic mutations. Researchers had almost 70 years of experience developing adenovirus-based vaccines, which are distinct from adenoviral vectors. The U.S. military, for instance, administered live Ad types 4 (Ad4) and 7 (Ad7) to recruits in the 1950s, according to an article in Cell.
An early severe safety event
But one of the first recipients of an Ad5-based gene therapy died after treatment in 1999. That recipient, Jesse Gelsinger, had suffered from a rare genetic liver disease. The adenoviral-vector treatment shows promise in some animal studies but caused severe adverse events in Gelsinger. “When Jesse got the vector, he suffered a chain reaction that the testing had not predicted — jaundice, a blood-clotting disorder, kidney failure, lung failure and brain death,” according to an article from The New York Times. The patient had received a dose of 38 trillion viruses.
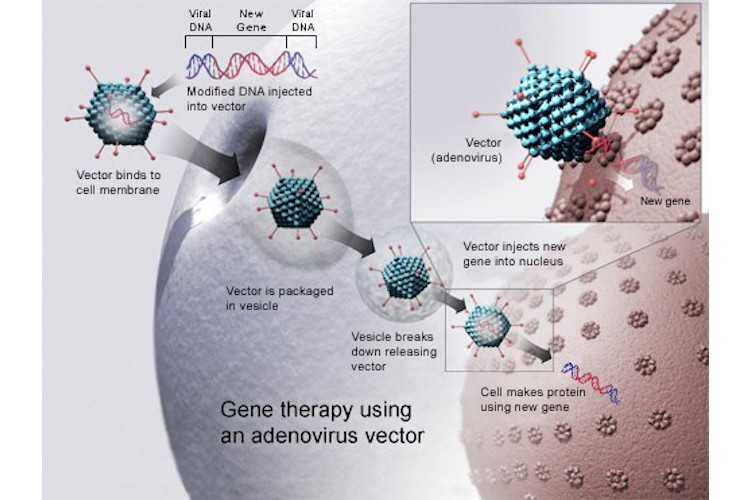
Researchers have explored adenovirus vectors for both gene therapy and vaccines. Image courtesy of Wikipedia
While the vector generally seemed to work well in animal studies, three monkeys receiving it died of a blood-clotting disorder accompanied by severe liver inflammation. The monkeys had received an exceptionally high dose of the adenovirus vector.
Further generations of adenoviral vectors
Following this period, scientists backed away from using high doses of adenoviral vectors for gene therapy. But researchers reasoned that engineered adenoviruses that cannot replicate would be ideal for vaccines.
The potential of adenovirus vectors for gene therapy brightened. Next-generation vectors have improved safety and immunogenicity.
The research spurred several developers to explore the use of the adenoviral vector platform for vaccines.
Still a new technology
Before the introduction of adenovirus-vectored vaccines from AstraZeneca, Johnson & Johnson and others, however, such vaccines had not found broad use.
“There is only one [adenoviral vaccine] that has been approved so far,” said Navin Jacob, a UBS analyst referring to the Ebola vaccine from Johnson & Johnson that has won approval in Europe in 2020.
The Ad26 vector of the Johnson & Johnson COVID-19 vaccine has also been used in investigational vaccines for HIV, Zika, filovirus, HPV, malaria and respiratory syncytial virus. “As of December 2020, Ad26-based vaccines have been used to vaccinate 193,831 participants in clinical studies and vaccination programs and have exhibited acceptable clinical safety,” concluded a review of the Johnson & Johnson COVID-19 vaccine from UT Southwestern researchers.
In the Phase 3 trials for the AstraZeneca and Johnson & Johnson vaccines, reports of serious adverse events transverse myelitis led to pauses in the clinical trials. Investigators later deemed those events to be unrelated to the vaccines.
Investigators also noted a numerical imbalance of thromboembolic events between vaccine and placebo recipients. Investigators determined that there were insufficient data to establish a causal relationship between the vaccine and the events.
Risk from adenoviral vector vaccines remains small overall
The overall risk of blood-clotting events from adenoviral vector COVID-19 vaccines “remains relatively low versus the risk associated with COVID-19,” Jacob said. “We’re talking about extremely rare events.”
There have been at least 222 suspected cases of such blood clots among 34 million vaccine recipients in Europe. More than 30 have died. In the U.S., there have been six such cases out of 7 million vaccinated individuals, including one death.
Still, there is reason to suspect such blood clotting events could be linked to adenoviral-vector vaccines. “The mechanism is not clear, but the events do appear to be very similar to the immune-mediated safety side effect associated with heparin-induced thrombocytopenia plus thrombosis,” Jacob said.
The problem might be specific to these adenoviral vector vaccines, but it is also unclear whether the issue will also apply to other similar vaccines in development. “It is not clear until the mechanism by which this is happening is clarified, it’s very hard to say,” Jacob said.