
Pfizer-BioNTech vaccine image courtesy of Wikipedia
While vaccines remain the most powerful tool in achieving herd immunity for COVID-19, mass-vaccination has thus far proven more challenging than anticipated in many parts of the world. There are expected challenges, such as dealing with the subarctic storage requirements (–112º to –76º F) of the BNT162b2 vaccine from Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX). But the vaccine rollout is posing a series of challenges, many of which have been hard to anticipate.
1. Lack of standardization
One factor slowing COVID-19 vaccine distribution is confusion. “One of our biggest challenges right now is just lack of standardization across the U.S.,” said Marsha Flores Harris, product manager for FDB Prizm, a knowledge base platform from privately-held First Databank.
Not only is there variability in the storage requirements for the vaccines likely to be used in 2021, but the vaccines have different supply chains. The state of Ohio has tapped Cardinal Health (NYSE: CAH) for vaccine distribution. The Centers for Disease Control (CDC) is working with McKesson to distribute vaccines. “Most of the Moderna and the Pfizer vaccines are going to flow through McKesson through their centralized [distribution centers],” Harris said. From there, the company will ship vaccines out to their customers. The needs of those customers can vary based on where they are located. “ The biggest issue is just getting the vaccine out to each state based on their own requirements because there’s so much variability.”
2. Demand surge for ultracold freezers

Image courtesy of Stirling Ultracold
Ultracold freezers have become a hot commodity during the pandemic — in large part because of the storage requirements of the Pfizer-BioNTech vaccine. Healthcare facilities, states and cities aren’t the only ones driving up demand. The carmaker Ford, for instance, ordered 12 ultracold freezers late last year in a campaign to offer employees voluntary vaccinations.
“Some of the ultracold freezer manufacturers are kicking these things out by the dozens rather than the thousands,” Harris said. “There’s not a whole lot of stock out there.”
Some hospitals in Massachusetts, for instance, have resorted to borrowing such freezers.
The Moderna vaccine has more forgiving storage requirements than Pfizer’s while the AstraZeneca (LON:AZN) vaccine is a more traditional refrigerated vaccine.
3. Shortage of transport coolers
Transport coolers, which resemble ice chests but carry vaccines, are in short supply. “They are backordered,” Harris said. “I’ve seen shortages of anywhere from three weeks out to the end of February.
4. Temperature can vary within containers and freezers
It is possible to have temperature variations within refrigerators or freezers used to store vaccines. For a refrigerated vaccine, for example, it is possible for some vials to be closer to the coolant than others, resulting in some of the vaccines getting too cold. “Conversely, if your vials are very far away from that coolant, or maybe at the top of the pack, or in a hot spot, that time you run the risk of the heat event,” said Chris Caulfield, vice president, operations at TempTime, a subsidiary of Zebra Technologies (NSDQ:ZBRA). The company offers vial-level monitoring of vaccine temperature.
Large containers, refrigerators or freezers are more likely to have temperature variability. “You’re more likely to have hot spots or cold spots,” Caulfield said.
5. A surge in dry ice demand
While the press outlets have warned of a pending dry ice shortage, there’s little evidence of a short-term problem, Harris said. “If you talk to FedEx and UPS, which make their own dry ice, they say there’s plenty of dry ice to go around.”
6. Airline restrictions for vaccine transportation
In the U.S., vaccine logistics relies on a public-private partnership with companies like McKesson, FedEx and UPS playing important roles. The Wall Street Journal also reported that Pfizer is partnering with United Airlines on vaccine delivery.
The plan requires charter flights because of the high volumes of dry ice involved in transporting the Pfizer-BioNTech vaccine. The Federal Aviation Administration (FAA) classifies dry ice as a dangerous good and limits the volume of dry ice allowed on flights. United Airlines has asked the FAA to allow it to carry more dry ice than usual to support the ultracold temperatures the Pfizer vaccine requires. FAA agreed to allow the airline to carry 15,000 pounds per flight — five times the normal allowable amount.
American Airlines, FedEx, DHL, UPS and Lufthansa are also involved in air transport of COVID-19 vaccines.
7. Temperature excursions are a concern
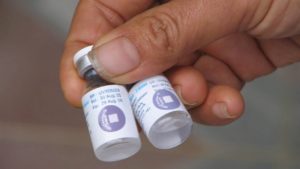
Vial-level vaccine temperature monitoring from TempTime. Image courtesy of Zebra Technologies
The impact of COVID-19 vaccines getting too hot or cold during storage is unclear, but such temperature excursions likely affect efficacy. “I think the worst result that could happen is if someone gets a vaccine that has been exposed to a temperature event and the vaccine is not effective,” Caulfield said.
One complication is that there are no clear signs from vaccines that they have been exposed to high temperatures. “When a vaccine is exposed to heat, it’s invisible,” Caulfield said. “There’s no physical change to the vaccine.”
8. Impact on conventional childhood immunizations
While the COVID-19 pandemic has inspired unprecedented vaccine development efforts, traditional childhood vaccination programs have suffered in the past year. “Many of the large global vaccination programs were suspended or postponed until later 2020 because of the severe impact of COVID-19 on a global basis,” Caulfield said. “There’s alot of discussion about the impact of children missing their regularly scheduled vaccination, whether that’s for polio, measles, diphtheria, tetanus, pertussis or whatever. There’s lots of children running around and need to get their vaccinations again.”